Microencapsulation of Vanilla Oleoresin (V. planifolia Andrews) by Complex Coacervation and Spray Drying: Physicochemical and Microstructural Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. V. planifolia Andrews Oleoresin Extraction with SC-CO2
2.3. Complex Coacervates (CCs)
2.3.1. Experimental Design
2.3.2. Preparation of the CCs
2.3.3. Determination of ζ-Potential, Particle Size and Polydispersity Index (PDI) of CCs
2.4. Spray Drying of the Optimal CCs
2.5. Characterization of the Spray-Dried Microcapsules
2.5.1. Moisture Content and Water Activity (aw)
2.5.2. Quantification of Surface Vanilla Oleoresin and Retention and Microencapsulation Efficiencies
2.6. Fourier Transform Infrared Spectroscopy (FT-IR)
2.7. Environmental Scanning Electron Microscopy (ESEM)
2.8. Confocal Scanning Laser Microscopy (CLSM)
2.9. Chromaticity Analysis with Digital Image Analysis
2.10. Statistical Analyses
3. Results and Discussion
3.1. Effect of CH–GA Interaction and pH on Complex Coacervation
3.2. Chemical and Microstructural Characterization of CCs and Spray-Dried Microcapsules
3.2.1. Fourier Transform Infrared Spectroscopy (FT-IR)
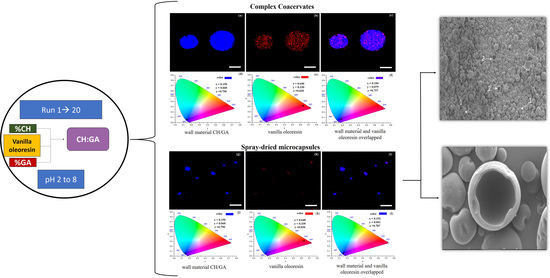
3.2.2. Confocal Scanning Laser Microscopy (CLSM) and Chromaticity
3.2.3. Physicochemical Characterization of Spray-Dried Microcapsules
3.2.4. Morphology of Spray-Dried Microcapsules
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bory, S.; Lubinsky, P.; Risterucci, A.M.; Noyer, J.L.; Grisoni, M.; Duval, M.F.; Besse, P. Patterns of introduction and diversification of Vanilla planifolia (Orchidaceae) in Reunion Island (Indian Ocean). Am. J. Bot. 2008, 95, 805–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dignum, M.J.W.; Kerler, J.; Verpoorte, R. Vanilla production: Technological, chemical, and biosynthetic aspects. Food Rev. Int. 2001, 17, 199–219. [Google Scholar] [CrossRef]
- Castillo-Ruz, M.C.; Guillermo-Alcocer, C.G.; Bojórquez-Gamboa, R.R.; Rocha-Uribe, J.A. Extraction of vanilla oleoresin (Vanilla planifolia Andrews) with supercritical CO2. Tecnol. Cienc. Educ. 2011, 26, 80–84. [Google Scholar]
- de Guzman, C.C.; Zara, R.R. Vanilla. In Handbook of Herbs and Spices, 2nd ed.; Peter, K.V., Ed.; Woodhead Publishing in Food Science, Technology and Nutrition: Cambridge, UK, 2012; pp. 547–589. [Google Scholar]
- Hernández-Fernández, M.Á.; Rojas-Avila, A.; Vazquez-Landaverde, P.A.; Cornejo-Mazón, M.; Dávila-Ortiz, G. Volatile compounds and fatty acids in oleoresins from Vanilla planifolia Andrews obtained by extraction with supercritical carbon dioxide. CYTA J. Food 2019, 17, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Jimenes, G.C.; Vargas-García, A.; Espinoza-Pérez, D.J.; Salgado-Cervantes, M.A.; Robles-Olvera, V.J.; García-Alvarado, M.A. Mass transfer during vanilla beans solid liquid extraction: Effect of extraction method. Food Bioprocess Technol. 2013, 10, 2640–2650. [Google Scholar] [CrossRef]
- Dunphy, P.; Bala, K. The Role of Lipids in Vanilla Beans and their Transformation during Curing. A review of the effects, including flavor formation. Perfum. Flavor. 2014, 39, 20–28. [Google Scholar]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Peng, Z.; Li, J.; Li, S.; Kong, L.; Li, P.; Qinghuang, W. Development and evaluation of novel flavour microcapsules containing vanilla oil using complex coacervation approach. Food Chem. 2014, 145, 272–277. [Google Scholar] [CrossRef]
- Noshad, M.; Mohebbi, M.; Shahidi, F.; Koocheki, A. Effect of layer-by-layer polyelectrolyte method on encapsulation of vanillin. Int. J. Biol. Macromol. 2015, 81, 803–808. [Google Scholar] [CrossRef]
- Ocampo-Salinas, I.O.; Jiménez-Aparicio, A.; Perea-Flores, M.J.; Tapia-Ochoategui, A.; Salgado-Cruz, M.P.; Jimenez-Martinez, C.; Tellez-Medina, D.I.; Dávila-Ortiz, G. High-pressure homogenization and maltodextrins mixtures to microencapsulate vanilla (Vanilla planifolia) extract through freeze-drying. Rev. Mex. Ing. Quim. 2017, 16, 131–146. [Google Scholar]
- Calva-Estrada, S.J.; Mendoza, M.R.; García, O.; Jiménez-Fernández, V.M.; Jiménez, M. Microencapsulation of vanilla (Vanilla planifolia Andrews) and powder characterization. Powder Technol. 2018, 323, 416–423. [Google Scholar] [CrossRef]
- Gomez-Estaca, J.; Comunian, T.; Montero, P.; Ferro-Furtado, R.; Favaro-Trindade, C. Encapsulation of an astaxanthin-containing lipid extract from shrimp waste by complex coacervation using a novel gelatin–cashew gum complex. Food Hydrocoll. 2018, 61, 155–162. [Google Scholar] [CrossRef]
- Taneja, A.; Singh, H. Challenges for the delivery of long-chain n-3 fatty acids in functional foods. Annu. Rev. Food Sci. Technol 2012, 3, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; Turgeon, S.L. Protein/polysaccharide complexes and coacervates in food systems. J. Colloid Interface Sci. 2011, 167, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Niu, D.; Zhang, H.; Chang, C.; Gu, L.; Su, Y.; Yang, Y. Ovalbumin/gum Arabic-stabilized emulsion: Rheology, emulsion characteristics, and Raman spectroscopic study. Food Hydrocoll. 2016, 52, 607–614. [Google Scholar] [CrossRef]
- Tan, C.; Xie, J.; Zhang, X.; Cai, J.; Xia, S. Polysaccharide-based nanoparticles by chitosan and gum arabic polyelectrolyte complexation as carriers for curcumin. Food Hydrocoll. 2016, 57, 236–245. [Google Scholar] [CrossRef]
- Fang, Z.X.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, H.; Hu, X.; Bao, S.; Huang, H. Synthesis and release studies of microalgal oil-containing microcapsules prepared by complex coacervation. Colloids Surf. B Biointerfaces 2012, 89, 61–66. [Google Scholar] [CrossRef]
- Souza, C.; Rojas, E.; Melo, N.; Gaspar, A.; Lins, J. Complex coacervates obtained from interaction egg yolk lipoprotein and polysaccharides. Food Hydrocoll. 2013, 30, 375–381. [Google Scholar] [CrossRef]
- Moschakis, T.; Murray, B.S.; Biliaderis, C.G. Modifications in stability and structure of whey protein-coated o/w emulsions by interacting chitosan and gum arabic mixed dispersions. Food Hydrocoll. 2010, 24, 8–17. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Báez-González, J.G.; Cruz-Sosa, F.; Vernon-Carter, E. Gum Arabic–chitosan complex coacervation. Biomacromolecules 2007, 8, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Avadi, M.R.; Sadeghi, A.M.M.; Mohammadpour, N.; Abedin, S.; Atyabi, F.; Dinarvand, R.; Rafiee-Tehrani, M. Preparation and characterization of insulin nanoparticles using chitosan and Arabic gum with ionic gelation method. Nanomedicine 2010, 6, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Andrews, H.; Enríquez-Ramírez, K.; García-Márquez, E.; Ramírez-Santiago, C.; Lobato-Calleros, C.; Vernon-Carter, J. Interrelationship between the zeta potential and viscoelastic properties in coacervates complexes. Carbohydr. Polym. 2013, 95, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Butstraen, C.; Salaün, F. Preparation of microcapsules by complex coacervation of gum Arabic and chitosan. Carbohydr. Polym. 2014, 99, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, N.D.; Grosso, C.R.F.; Rabelo, R.S.; Hubinger, M.D.; Prata, A.S. Comparison of microparticles produced with combinations of gelatin, chitosan and gum Arabic. Carbohydr. Polym. 2018, 196, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, R.S.; Tavares, G.M.; Prata, A.S.; Hubinger, M.D. Complexation of chitosan with gum Arabic, sodium alginate and κ-carrageenan: Effects of pH, polymer ratio and salt concentration. Carbohydr. Polym. 2019, 223, 115–120. [Google Scholar] [CrossRef]
- Vuillemin, M.E.; Michaux, F.; Muniglia, L.; Linder, M.; Jasniewski, J. Gum Arabic and chitosan self-assembly: Thermodynamic and mechanism aspects. Food Hydrocoll. 2019, 96, 463–474. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromols 2019, 121, 1276–1286. [Google Scholar] [CrossRef]
- Woo, M.W.; Bhandari, B. Spray drying for food powder production. In Handbook of Food Powders: Processes and Properties; Bhandari, B., Bansal, N., Zhang, M., Schuck, P., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 29–56. [Google Scholar] [CrossRef]
- Ocampo-Salinas, I.O.; Gómez-Aldapa, C.A.; Castro-Rosas, J.; Vargas-León, E.A.; Guzmán-Ortiz, F.A.; Calcáneo-Martínez, N.; Falfán-Cortés, R.N. Development of wall material for the microencapsulation of natural vanilla extract by spray drying. Cereal Chem. 2020, 97, 555–565. [Google Scholar] [CrossRef]
- Aguirre-Alonso, R.O.; Morales-Guillermo, M.; Salgado-Cervantes, M.A.; Robles-Olvera, V.J.; García-Alvarado, M.A.; Rodríguez-Jimenes, G.C. Effect of process variables of spray drying employing heat pump and nitrogen on aromatic compound yield in powders obtained from vanilla (Vanilla planifolia Andrews) ethanolic extract. Dry. Technol. 2019, 37, 1806–1820. [Google Scholar] [CrossRef]
- Hasanvand, E.; Rafe, A. Development of vanillin/β-cyclodextrin inclusion microcapsules using flax seed gum-rice bran protein complex coacervates. Int. J. Biol. Macromol. 2019, 15, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Ávila, A.; Pimentel-Rodas, A.; Rosales-García, T.; Dávila-Ortiz, G.; Galicia-Luna, L.A. Solubility of Binary and Ternary Systems Containing Vanillin and Vanillic Acid in Supercritical Carbon Dioxide. J. Chem. Eng. 2016, 61, 3225–3232. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, M.N.; Kathiravan, T.; Moses, J.A.; Anandharamakrishnan, C. Influence of spray-drying conditions on microencapsulation of fish oil and chia oil. Dry. Technol. 2020, 38, 279–292. [Google Scholar] [CrossRef]
- Tan, S.; Zhong, C.; Langrish, T. Encapsulation of caffeine in spray-dried micro-eggs for controlled release: The effect of spray-drying (cooking) temperature. Food Hydrocoll. 2020, 108, 105979. [Google Scholar] [CrossRef]
- Lee, M.H. Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists International: Arlington, VA, USA, 1995. [Google Scholar]
- Eratte, D.; Wang, B.; Dowling, K.; Barrow, C.J.; Adhikari, B.P. Complex coacervation with whey protein isolate and gum arabic for the microencapsulation of omega-3 rich tuna oil. Food Funct. 2014, 5, 2743–2750. [Google Scholar] [CrossRef]
- Liu, S.; Low, N.H.; Nickerson, M.T. Entrapment of Flaxseed Oil within Gelatin-Gum Arabic Capsules. J. Am. Oil Chem. Soc. 2010, 87, 809–815. [Google Scholar] [CrossRef]
- Yang, X.; Gao, N.; Hu, L.; Li, J.; Sun, Y. Development and evaluation of novel microcapsules containing poppy-seed oil using complex coacervation. J. Food Eng. 2015, 161, 87–93. [Google Scholar] [CrossRef]
- Quintanilla-Carvajal, M.X.; Meraz-Torres, L.S.; Alamilla-Beltrán, L.; Chanona-Pérez, J.J.; Terres-Rojas, E.; Hernández-Sánchez, H.; Jiménez-Aparicio, A.R.; Gutiérrez-López, G.F. Morphometric characterization of spray-dried microcapsules before and after α-tocopherol extraction. Rev. Mex. Ing. Quim. 2011, 10, 301–312. [Google Scholar]
- CIE. Commission Internationale De l’Éclairage Proceedings; Cambridge University Press: Cambridge, UK, 1931. [Google Scholar]
- Quintanilla-Carvajal, M.X.; Hernández-Sánchez, H.; Alamilla-Beltrán, L.; Zepeda-Vallejo, G.; Jaramillo-Flores, M.E.; de Jesús Perea-Flores, M.; Gutiérrez-López, G.F. Effects of microfluidization process on the amounts and distribution of encapsulated and non-encapsulated α-tocopherol microcapsules obtained by spray drying. Food Res. Int. 2014, 63, 2–8. [Google Scholar] [CrossRef]
- Tsai, R.-Y.; Chen, P.-W.; Kuo, T.-Y.; Lin, C.-M.; Wang, D.-M.; Hsien, T.-Y.; Hsieh, H.-J. Chitosan/pectin/gum Arabic polyelectrolyte complex: Process-dependent appearance, microstructure analysis and its application. Carbohydr. Polym. 2014, 101, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Andrews, H.; Sandoval-Castilla, O.; Vázquez-Torres, H.; Vernon-Carter, E.J.; Lobato-Calleros, C. Determination of the gum Arabic–chitosan interactions by Fourier Transform Infrared Spectroscopy and characterization of the microstructure and rheological features of their coacervates. Carbohydr. Polym. 2010, 79, 541–546. [Google Scholar] [CrossRef]
- Helgason, T.; Gislason, J.; McClements, D.J.; Kristbergsson, K.; Weiss, J. Influence of molecular character of chitosan on the adsorption of chitosan to oil droplet interfaces in an in vitro digestion model. Food Hydrocoll. 2009, 23, 2243–2253. [Google Scholar] [CrossRef]
- Rojas-Moreno, S.; Cárdenas-Bailón, F.; Osorio-Revilla, G.; Gallardo-Velázquez, T.; Proal-Nájera, J. Effects of complex coacervation-spray drying and conventional spray drying on the quality of microencapsulated orange essential oil. J. Food Meas. Charact. 2017, 12, 650–660. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Groups Frequencies, Tables and Charts; John Wiley and Sons: Chichester, UK, 2001; ISBN 9780470093078. [Google Scholar]
- Abreu, F.O.M.d.S.; Silva, N.A.d.; Sipauba, M.d.S.; Pires, T.F.M.; Bomfim, T.A.; Monteiro Junior, O.A.d.C.; Forte, M.M.d.C. Chitosan and gum arabic nanoparticles for heavy metal adsorption. Polímeros 2018, 28, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Zou, Q.; Li, J.; Li, Y. Preparation and characterization of vanillin-crosslinked chitosan therapeutic bioactive microcarriers. Int. J. Biol. Macromols 2015, 79, 736–747. [Google Scholar] [CrossRef]
- Santiago de Alvarenga, E. Characterization and Properties of Chitosan. In Biotechnology of Biopolymers; Elnashar, M., Ed.; InTech: Rijeka, Croatia, 2011; Volume 91, pp. 95–102. [Google Scholar]
- Balanta, D.; Grande, C.; Zuluaga, F. Extracción, identificación caracterización de quitosano del micelio de Aspergillus niger y sus aplicaciones como material bioadsorbente en el tratamiento de aguas. Rev. Iberoam. Polim. 2010, 11, 297–316. [Google Scholar]
- Kumirska, J.; Czerwicka, M.; Kaczynski, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods of Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [Green Version]
- Drusch, S.; Diekmann, S. Microencapsulation by Spray Drying. In Handbook of Encapsulation and Controlled Release; Munmaya, M., Ed.; Taylor & Francis, CRC Press: Boca Raton, FL, USA, 2016; pp. 35–44. [Google Scholar] [CrossRef]
- Yeo, Y.; Bellas, E.; Firestone, W.; Langer, R.; Kohane, D.S. Complex coacervates for thermally sensitive controlled release of flavor compounds. J. Agric. Food Chem. 2005, 53, 7518–7525. [Google Scholar] [CrossRef]
- Dong, Z.J.; Toure, A.; Jia, C.S.; Zhang, X.M.; Xu, S.Y. Effect of processing parameters on the formation of spherical multinuclear microcapsules encapsulating peppermint oil by coacervation. J. Microencapsul. 2007, 24, 634–646. [Google Scholar] [CrossRef]
- Bosnea, L.A.; Moschakis, T.; Biliaderis, C.G. Complex Coacervation as a Novel Microencapsulation Technique to Improve Viability of Probiotics under Different Stresses. Food Bioprocess Technol. 2014, 7, 2767–2781. [Google Scholar] [CrossRef]
- Gulão, E.S.; de Souza, C.J.; Andrade, C.T.; Garcia-Rojas, E.E. Complex coacervates obtained from peptide leucine and gum arabic: Formation and characterization. Food Chem. 2016, 194, 680. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Zhao, Y.; Ding, J.; Lin, S. Investigation on complex coacervation between fish skin gelatin from cold-water fish and gum arabic: Phase behavior, thermodynamic, and structural properties. Food Res. Int. 2018, 107, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Nori, M.P.; Favaro-Trindade, C.S.; Matias de Alencar, S.; Thomazini, M.; de Camargo Balieiro, J.C.; Contreras-Castillo, C.J. Microencapsulation of propolis extract by complex coacervation. LWT 2011, 44, 429–435. [Google Scholar] [CrossRef]
- Rodríguez, S.D.; Wilderjans, T.F.; Sosa, N.; Bernik, D.L. Image Texture Analysis and Gas Sensor Array Studies Applied to Vanilla Encapsulation by Octenyl Succinic Anhydride Starches. J. Food Res. 2013, 2, 36. [Google Scholar] [CrossRef] [Green Version]
- Vega, C.; Roos, Y.H. Invited review: Spray-dried dairy and dairy-like emulsions compositional. Int. J. Dairy Sci. 2006, 89, 383–401. [Google Scholar] [CrossRef] [Green Version]
- Roldan-Cruz, C.; Carmona-Ascencio, J.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Electrical impedance spectroscopy for monitoring the gum Arabic–chitosan complexation process in bulk solution. Colloid Surfaces A 2016, 495, 125–135. [Google Scholar] [CrossRef]
- Tavares, L.; Zapata-Noreña, C.P. Encapsulation of garlic extract using complex coacervation with whey protein isolate and chitosan as wall materials followed by spray drying. Food Hydrocoll. 2019, 89, 360–369. [Google Scholar] [CrossRef]
- Ghannam, H.E.; Talab, A.S.; Dolgano, N.V.; Husse, A.; Abdelmagui, N.M. Characterization of chitosan extracted from different crustacean shell wastes. JApSc 2016, 16, 454–461. [Google Scholar] [CrossRef]
- Ciofani, G.; Mattoli, V. Boron Nitride Nanotubes in Nanomedicine; William Andrew: Norwich, NY, USA, 2016. [Google Scholar] [CrossRef]
- Porras-Saavedra, J.; Alamilla-Beltrán, L.; Lartundo-Rojas, L.; de Jesús Perea-Flores, M.; Yáñez-Fernández, J.; Palacios-González, E.; Gutiérrez-López, G.F. Chemical components distribution and morphology of microcapsules of paprika oleoresin by microscopy and spectroscopy. Food Hydrocoll. 2018, 81, 6–14. [Google Scholar] [CrossRef]
- Bhusari, S.N.; Muzaffar, K.; Kumar, P. Effect of Carrier agents on physical and microstructural properties of spray dried tamarind pulp powder. Powder Technol. 2014, 266, 354–364. [Google Scholar] [CrossRef]



| Run | Complex Coacervation Conditions | Results | |||||
|---|---|---|---|---|---|---|---|
| %CH + %GA | CH:GA 1 | pH | ζ-Potential (mV) | Particle Size (nm) | PDI | ||
| 1 | 0.63 | 3.00 | 1:4.8 | 5.00 | −6.63 | 1635.33 | 0.88 |
| 2 | 0.25 | 5.00 | 1:20 | 8.00 | −23.70 | 299.77 | 0.43 |
| 3 | 0.63 | 1.00 | 1:1.6 | 5.00 | 16.37 | 123.67 | 0.27 |
| 4 | 0.63 | 3.00 | 1:4.8 | 2.00 | 12.17 | 118.10 | 0.42 |
| 5 | 0.63 | 3.00 | 1:4.8 | 5.00 | −6.91 | 1635.33 | 0.88 |
| 6 | 0.63 | 3.00 | 1:4.8 | 5.00 | −6.42 | 1635.33 | 0.88 |
| 7 | 1.00 | 3.00 | 1:3 | 5.00 | 18.80 | 309.70 | 0.45 |
| 8 | 0.25 | 1.00 | 1:4 | 8.00 | −3.56 | 1591.67 | 0.62 |
| 9 | 0.63 | 3.00 | 1:4.8 | 5.00 | −6.65 | 1635.33 | 0.88 |
| 10 | 0.25 | 5.00 | 1:20 | 2.00 | 0.56 | 1936.38 | 0.27 |
| 11 | 1.00 | 5.00 | 1:5 | 8.00 | −15.83 | 361.60 | 0.46 |
| 12 | 0.63 | 3.00 | 1:4.8 | 5.00 | −7.82 | 1635.33 | 0.88 |
| 13 | 0.63 | 5.00 | 1:8 | 5.00 | −19.23 | 85.86 | 0.39 |
| 14 | 1.00 | 1.00 | 1:1 | 8.00 | 32.60 | 255.43 | 0.42 |
| 15 | 0.63 | 2.50 | 1:4 | 8.00 | −13.43 | 442.17 | 0.57 |
| 16 | 0.25 | 1.00 | 1:4 | 2.00 | 19.70 | 136.00 | 0.27 |
| 17 | 0.63 | 3.00 | 1:4.8 | 5.00 | −6.63 | 1635.33 | 0.88 |
| 18 | 1.00 | 5.00 | 1:5 | 2.00 | 9.59 | 527.07 | 0.56 |
| 19 | 0.25 | 3.00 | 1:12 | 5.00 | −23.77 | 359.93 | 0.51 |
| 20 | 1.00 | 1.00 | 1:1 | 2.00 | 29.25 | 226.60 | 0.44 |
| Formulation | %CH | %GA | CH:GA Ratio | pH | ζ-Potential (mV) |
|---|---|---|---|---|---|
| 1 | 0.9 | 3.52 | 1:4 | 6.11 | −2.93 × 10−5 |
| 2 | 0.83 | 3.74 | 1:4.5 | 5.1 | −2.96 × 10−5 |
| 3 | 0.85 | 3.46 | 1:4 | 5.83 | −4.95 × 10−5 |
| 4 | 0.85 | 4.29 | 1:5 | 4.06 | −5.42 × 10−5 |
| 5 | 0.7 | 3.39 | 1:4.8 | 4.69 | 8.44 × 10−5 |
| 6 | 0.8 | 4.01 | 1:5 | 4.24 | −8.44 × 10−5 |
| 7 | 0.47 | 2.36 | 1:5 | 5.02 | −9.59 × 10−5 |
| 8 | 0.34 | 1.7 | 1:5 | 5.29 | −9.64 × 10−5 |
| 9 | 0.81 | 3.18 | 1:4 | 6.1 | 1.33 × 10−5 |
| 10 | 0.55 | 1.78 | 1:3 | 6.89 | −1.26 × 10−5 |
| Bond | –OHv, –NHv | C–Hv | C=Nv | C=Ov, –OHδ | C–Ov |
|---|---|---|---|---|---|
| Wavenumber (cm−1) | 3270 | 2926 | 1568 | 1411 | 1022 |
| % Transmittance vanilla oleoresin | 94.90 | 94.63 | 77.76 | 83.03 | 72.55 |
| % Transmittance CH-GA | 77.95 | 89.86 | 88.90 | 86.07 | 31.16 |
| % Transmittance Complex Coacervates | 86.79 | 88.58 | 65.36 | 63.86 | 42.43 |
| % Transmittance Spray-Dried Microcapsules | 94.42 | 95.16 | 80.19 | 79.98 | 70.49 |
| % Difference CCs and the Microcapsules | 7.63 | 6.58 | 14.83 | 16.12 | 28.06 |
| VO:WM | aw | %Moisture | %RE | %SVO | %EE |
|---|---|---|---|---|---|
| 1:2.5 | 0.13 ± 0.01 | 3.80 ± 0.02 | 84.89 ± 1.94 | 15.69 ± 0.41 | 69.20 ± 1.79 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Fernández, M.Á.; García-Pinilla, S.; Ocampo-Salinas, O.I.; Gutiérrez-López, G.F.; Hernández-Sánchez, H.; Cornejo-Mazón, M.; Perea-Flores, M.d.J.; Dávila-Ortiz, G. Microencapsulation of Vanilla Oleoresin (V. planifolia Andrews) by Complex Coacervation and Spray Drying: Physicochemical and Microstructural Characterization. Foods 2020, 9, 1375. https://doi.org/10.3390/foods9101375
Hernández-Fernández MÁ, García-Pinilla S, Ocampo-Salinas OI, Gutiérrez-López GF, Hernández-Sánchez H, Cornejo-Mazón M, Perea-Flores MdJ, Dávila-Ortiz G. Microencapsulation of Vanilla Oleoresin (V. planifolia Andrews) by Complex Coacervation and Spray Drying: Physicochemical and Microstructural Characterization. Foods. 2020; 9(10):1375. https://doi.org/10.3390/foods9101375
Chicago/Turabian StyleHernández-Fernández, Miguel Ángel, Santiago García-Pinilla, Oswaldo Israel Ocampo-Salinas, Gustavo Fidel Gutiérrez-López, Humberto Hernández-Sánchez, Maribel Cornejo-Mazón, María de Jesús Perea-Flores, and Gloria Dávila-Ortiz. 2020. "Microencapsulation of Vanilla Oleoresin (V. planifolia Andrews) by Complex Coacervation and Spray Drying: Physicochemical and Microstructural Characterization" Foods 9, no. 10: 1375. https://doi.org/10.3390/foods9101375