3.1. Rheological Properties of GF Batter
The best-fit polynomial models for STIba and FIRba are presented in
Table 3. Results show that models of two-factor interaction were adequate to describe all of the GF batter properties. Moreover, according to equation Y
1, GG in interaction with XG and with HPMC, and XG in interaction with HPMC cause a decreasing effect on STIba—as can be deduced by the negative coefficients—although XG in interaction with GG boosts STIba to a greater extent than GG × HPMC and XG × HPMC interactions. According to equation Y
2, GG in interaction with XG and with HPMC, and XG in interaction with HPMC increase FIRba; although XG in interaction with GG appears stronger than GG × HPMC and XG × HPMC interactions.
To assess the significance of the GF batter properties’ models, results of ANOVA F-tests are shown in
Table 4. For STIba, the model (F-test
p-value <0.0001) was highly significant for describing the effects of the gums on batter stickiness. Likewise, the
p-values of the STIba model were all highly significant for the linear mixture terms and the interactions XG × GG, XG × HPMC, and GG × HPMC. The XG × HPMC interaction was a non-significant term which was eliminated from the FIRba model (
Table 3).
The model for STIba showed high
R2 (0.8269),
R2 adjusted (0.8185), and
R2 predicted (0.8067) values. On the contrary, low
R2 (0.6456),
R2 adjusted (0.6092), and
R2 predicted values (0.5575) were obtained for FIRba. In relation to the lack-of-fit test for GF batter properties, nearly all (except SCOba model) regression models yielded a significant lack-of-fit (
p < 0.05), which may indicate that a higher-order model is needed so as to approximate the true response surface [
24]. However, because this study attempts to explain the effect of the dependent variables using simple model responses, linear and two-way interaction terms were deemed sufficient. Although not shown here, cubic or quartic models turned the lack-of-fit values non-significant; however, these higher-order models are devoid of real explanation and, hence, were not considered as suitable in this study. The ‘prediction vs. residual’ plots built for each of the final models ailed to show outliers, indicating that models were parsimonious and with acceptable
R2 values.
As regards coefficient of variation (CV), both batter properties analyzed (STIba and FIRba) showed CV values below 10%, having stickiness as the lowest variability (CV = 2.51%). The values of AdPrec, were all higher than 4, indicating that the polynomial models of all GF batter properties could adequately discriminate among treatments. STIba had lower PRESS value than FIRba. For each of the GF batter properties, the model’s F-test was highly significant (
p < 0.001, in
Table 4). The linear mixture terms were significant for STIba. The interaction terms ‘XG × GG’, ‘XG × HPMC’, and ‘GG × HPMC’ were significant (
p < 0.05) for STIba; while for FIRba, ‘XG × HPMC’ was the only non-significant interaction term (therefore removed from the final model).
Two-dimensional plots (contour plots) and three-dimensional plots are given in
Figure 1 for the batter properties. The graphs are shown in five graduated color shades: blue for low values, light blue for intermediate values between low and medium, green for medium values, yellow for values between medium and high, and red for high values [
27]. Moreover, correlations between the dependent variable and the independent variables can be read off from the three-D response surface and two-D contour plots.
The response surfaces suggest that batters of lower stickiness were obtained with a combination of a high XG dose (0.60%) and intermediate doses of HPMC (3.00%) and GG (0.40%), while stickier batters were obtained with a combination of a high GG dose (0.60%), a low XG dose (0.04%) and a high-intermediate HPMC dose (3.36%;
Figure 1). For the back-extrusion property analyzed, firmer batters (higher FIRba values) were obtained with a combination of a high-intermediate dose of GG (0.48%) and a low-intermediate dose of HPMC (2.92%) and a high XG dose (0.60%). On the contrary, mixtures consisting of a high GG dose (0.60%), a high-intermediate HPMC dose (3.36%) and a low XG dose (0.04%) produced batters of lower FIRba value.
Although not directly comparable with this study, using a higher level of water (158%) in a mixture of rice/maize/soy (40:40:20), Sciarini et al. [
28] obtained lower batter firmness values, ranging from 336 to 679 g, for carrageenan (0.5%) and xanthan (0.5%), respectively. They concluded that the high molecular weight molecules of XG form complex aggregates through hydrogen bonds and polymer entanglements, resulting in a high Newtonian viscosity at low shear rates.
3.2. Physicochemical Quality Characteristics of GF Bread
Differences in height and crust appearance of the GF bread treatments can be appreciated in
Figure 2. The best-fit polynomial models for SVO, BLO, a
w, crust L* and crumb L* are presented in
Table 3. Results show that models of two-way interactions were adequate to describe all of the GF bread physicochemical properties. GG in interaction with XG and with HPMC causes a synergetic effect on SVO and a
w; although GG in interaction with HPMC appears stronger than with XG for SVO (equation Y
3). GG in interaction with HPMC appears slightly stronger than XG × GG interaction for a
w (equation Y
5). In agreement with equation Y
4, GG interacts with XG and with HPMC to cause a decreasing effect on BLO. GG in interaction with XG boosts BLO more strongly than GG × HPMC interaction. GG in interaction with XG and with HPMC causes a synergetic effect on crust L* (equation Y
6), and a decreasing effect on crumb L* (equation Y
7).
The ANOVA F-tests of the GF bread physicochemical models are shown in
Table 4. For SVO, the model (F-test
p-value <0.0001) was highly significant for describing the effects of the gums. Likewise, the
p-values of the SVO model were significant for the linear mixture terms and the interactions XG × GG and GG × HPMC. Since XG × HPMC was non-significant, it was eliminated from the models for SVO, BLO, a
w and crust L*; while the non-significant XG × GG term was removed as a predictor of crumb L*. The GG × HPMC interaction was non-significant for crumb L* (
Table 3).
The model for SVO showed very high R2 (0.9677), R2 adjusted (0.9666), and R2 predicted (0.9654) values. On the contrary, low R2 (0.2161), R2 adjusted (0.2015), and R2 predicted (0.1789) values were obtained for crust L*. Among the GF bread physicochemical properties, SVO presented the highest R2 value (0.9677) compared to BLO (0.7834) and aw (0.5987); while among the colorimetric parameters, crust L* had a moderate R2 value (0.4944) in comparison with crumb L* (0.2161). In relation to the lack-of-fit test for the GF bread physicochemical properties, nearly all (except BLO model) regression models yielded a highly significant lack-of-fit (p < 0.001).
In relation to CV, the lowest CV value was for a
w (0.07%), whereas the highest CV value was for baking loss (3.73%); moreover, all GF bread physicochemical properties showed CV values below 10%. Results inferior to 10% might be considered accurate, while values up to 20% are considered acceptable; however, for applications in agriculture, biotechnological processes, microbiology, and clinical protocols, the coefficients of variation are naturally high because of a wide dispersion in data [
29]. The values of AdPrec, were all higher than 4, indicating that the polynomial models of all bread GF physicochemical properties could adequately discriminate among treatments. Physicochemical properties of a
w, SVO and BLO had lower PRESS values than parameters of crust L* and crumb L*.
For each of the GF bread physicochemical properties, the model’s F-test was highly significant (
p < 0.001, in
Table 4). The linear mixture terms were significant (
p < 0.05) for all physicochemical properties. The interaction term ‘xanthan × guar’ was significant (
p < 0.05) for SVO, BLO, a
w and crust L*; interaction term ‘guar × HPMC’ was significant (
p < 0.05) for SVO, BLO, a
w and crust L*; while interaction term ‘xanthan × HPMC’ was highly significant (
p < 0.001) for crumb L*.
Table 3 displays only the statistically significant coefficients.
The response surfaces modeled suggest that bread loaves of high specific volume can be obtained with a combination of a high XG dose (0.60%) and intermediate doses of HPMC (3.08%) and GG (0.32%;
Figure 1). Lower moisture loss during baking were produced with a combination of a high XG dose (0.60%) and intermediate doses of GG (0.37%) and HPMC (3.03%). Lower crumb a
w values were obtained with a combination of a low HPMC dose (2.80%) and high doses of XG (0.60%) and GG (0.60%). Regarding the colorimetric properties of GF bread crust, lower crust L* values (i.e., darker crust) were produced with a combination of a low XG dose (0.04%), a high GG dose (0.60%) and a high-intermediate HPMC dose (3.36%). In relation to the crumb color, higher crumb L* values (i.e., lighter crumb) were obtained with a combination of a high XG dose (0.60%), and intermediate doses of GG (0.26%) and HPMC (3.14%).
Evaluating GF breads from different specialized supermarket sources, Matos and Rosell [
30] measured higher values of SVO (1.54–4.77 mL/g) and a
w (0.89–0.97 g) than those found in the present study; and found values of crumb L* (65.8–80.2) that were comparable to those measured in this study. Pongjaruvat et al. [
31] formulated GF breads varying pregelatinized tapioca starch doses, and obtained lower values for SVO (1.80–2.70 mL/g), similar values for crust L* (55.7–70), crumb L* (62.9–70.8), in a recipe of jasmine rice flour (100%), water content (80%), shortening (10%), sugar (9%), soy flour (10%), yeast (1.5%), and salt (1.5%). Bourekoua et al. [
3] studied different gums and water content (WC) in GF breadmaking formulations based on a mixture rice/field bean flour using a screening design, and obtained an optimum formula with a combination of 1.5% of gum arabic and 71.5% WC, obtaining a similar value for SVO (2.87 mL/g) and a darker crumb with a lower L* value (60.3). Demirkesen et al. [
4] studied different gums and emulsifiers in the elaboration of GF breads based on 100% rice flour and 150% water content, obtaining lower SVO values from 0.6 to 1.6 mL/g.
In this specific topic, considering that from a consumer, producer, and food safety points of view, larger-sized breads with lower baking losses, lower water activity crumbs, and darker crusts are preferred to smaller breads with higher aw and lighter crusts. Gum blends of low HPMC dose (2.80%) and high doses of XG (0.60%) and GG (0.60%) would appear as adequate since they lead to GF breads of higher SVO (2.85 mL/g) and lower values of BLO (12.6%), aw (0.982) and crust L* (68.7).
3.3. Textural Properties of GF Bread Crumb
According to
Table 5, some TPA properties were more discriminant than others: SPRbr, COHbr, and RESbr appeared as the most discriminant parameters capable of statistically distinguishing the different blends (
Table 5). Two-way interaction models were appropriate to describe the GF bread crumb textural properties of HARbr, ADHbr, COHbr, and RESbr (
Table 6). Moreover, in agreement with equations Y
8 and Y
9, GG in interaction with both XG and with HPMC causes a synergetic effect on HARbr and ADHbr, respectively; however, GG × XG has a stronger effect on HARbr than GGxHPMC, and the opposite is true for ADHbr. According to equation Y
10, GG in interaction with XG and with HPMC, and XG in interaction with HPMC cause a decreasing effect on COHbr; although XG in interaction with HPMC increases COHbr to a greater extent than XG × GG and GG × HPMC interactions, respectively. GG in interaction with XG has a stronger effect than in interaction with HPMC (equation Y
10). In relation to equation Y
11, GG in interaction with XG and with HPMC, and XG in interaction with HPMC causes a decreasing effect on RESbr; although XG in interaction with GG improves RESbr more strongly than both XG × HPMC and GG × HPMC interactions.
The ANOVA F-tests of textural properties of GF bread crumb models are shown in
Table 7. For HARbr, the model (F-test
p-value <0.0001) was highly significant for describing the individual effect (linear mixture terms) and the combined effects of interactions: XG × GG and GG × HPMC. In the prediction of HARbr, ADHbr, and RESbr, the XG × HPMC interaction was not significant. Likewise, the GG × HPMC interaction was eliminated from the RESbr model (
Table 6). The model for ADHbr showed high values of
R2 (0.7741),
R2 adjusted (0.7670) and
R2 predicted (0.7575). On the contrary, the lowest
R2 (0.2076),
R2 adjusted (0.189), and
R2 predicted (0.1513) values were obtained for RESbr. Among the GF bread crumb textural properties, ADHbr presented the highest
R2 value (0.7741) compared to HARbr (0.6602), COHbr (0.2849), and RESbr (0.2076). In relation to the lack-of-fit test for GF bread crumb textural properties, all regression models yielded a highly significant lack-of-fit (
p < 0.001), most likely due to data variability and the non-use of higher order terms, as explained before.
As regards CV, COHbr presented the lowest value (3.29%), while the highest one was for ADHbr (42.1%). Furthermore, COHbr and RESbr (6.49%) showed CV values below 10%, whereas HARbr (16.8%) and ADHbr presented CV values higher than 10%. The values of AdPrec, were all higher than 4, indicating that the polynomial models of all GF bread crumb textural properties could adequately discriminate among treatments. Crumb textural properties of COHbr and RESbr had lower PRESS values than HARbr and ADHbr. For each of the GF bread crumb textural properties, the model’s F-test and the linear mixture terms were highly significant (
p < 0.0001, in
Table 7). The interaction term ‘XG × HPMC’ was highly significant (
p < 0.0001) only for COHbr; and the interaction term ‘GG × HPMC’ was significant (
p < 0.05) for HARbr, ADHbr, and COHbr.
The response surfaces suggest that softer crumbs were obtained with a combination of a high-intermediate HPMC dose (3.36%), a high GG dose (0.60%) and a low XG dose (0.04%;
Figure 3). Mixtures consisting of a low XG dose (0.04%), a high-intermediate HPMC dose (3.36%) and a high dose of GG (0.60%) rendered lower ADHbr values. Higher crumb cohesiveness values can be obtained with combinations of high doses of XG (0.60%) and GG (0.60%) and a low HPMC dose (2.80%). Higher crumb resilience values were obtained with combinations of a high XG dose (0.60%), a low GG dose (0.04%), and a high-intermediate HPMC dose (3.36%).
According to Cornejo and Rosell [
32], high resilience values are preferred because it is a property related to the bread freshness and elasticity, as perceived by the consumers. A reduction in resilience characterizes loss of elasticity since that property indicates the ability of a material to return to its original shape after stressing. On the other hand, bread crumb with high cohesiveness is desirable because it forms a bolus, instead of disintegrating during mastication whereas low cohesiveness indicates increased susceptibility of the bread to fracture or crumble. Morreale et al. [
33] obtained, from GF bread elaborated with rice flour, lower values of hardness (145–267 g), comparable value of cohesiveness (0.748–0.805) and higher values of resilience (0.361–0.386). From GF bread elaborated with a base of rice flour and potato starch, Turkut et al. (2016) obtained a comparable crumb hardness (1672–3569 g) and cohesiveness (0.42–0.47), when they varied the levels of quinoa and buckwheat flours. In another study, Hager et al. [
34] obtained similar TPA hardness values (1916–6797 g). Bourekoua et al. [
3] obtained softer crumbs (1523 g); likewise, Demirkesen et al. [
4] obtained lower firmness (i.e., hardness) values from 25 to 255 g.
In this specific topic, since from a consumer standpoint, softer and more cohesive and elastic crumbs are preferred than harder and stiffer crumbs, combinations of a high HPMC dose (3.60%), a low XG dose (0.04%), and an intermediate GG dose (0.36%) would be appropriate to obtain GF bread crumbs of higher values of COHbr (0.433) and RESbr (0.167) and lower values of HARbr (2362 g) and ADHbr (−163 g·s).
3.4. Image Analysis of GF Bread Crumb Grain Features
Differences in crumb grain features of the GF bread treatments can be appreciated in
Figure 4. All of the crumb grain features were appropriately described by the response surface models (
Table 6). Furthermore, according to equation Y
12, GG in interaction with XG causes a decreasing effect on MCA. GG in interaction with XG and with HPMC causes a synergetic effect on CDE (equation Y
13), while XG in interaction with HPMC causes an opposite effect on CDE. GG interacts with XG to affect CDE to a greater extent than GG × HPMC and XG×HPMC do. According to equation Y
14, GG in interaction with XG and with HPMC causes a decreasing effect on VFR; although GG in interaction with XG has a stronger effect than in interaction with HPMC.
The ANOVA F-tests of the GF bread crumb grain features’ models are shown in
Table 7. For crumb cell density, the model (F-test
p-value <0.0001) was highly significant for describing the individual effect (linear mixture terms) and the combined effects of interactions of XG × GG, XG × HPMC and GG × HPMC. Since XG × HPMC interaction was non-significant, it was eliminated from the VFR model (
Table 6). The model for VFR showed high values of
R2 (0.6919),
R2 adjusted (0.6871), and
R2 predicted (0.6812). On the contrary, the lowest
R2 (0.6513),
R2 adjusted (0.6445), and
R2 predicted (0.6337) values were obtained for CDE. Among the GF bread crumb grain features, VFR presented the highest
R2 value (0.6919) in comparison with MCA (0.6522) and CDE (0.6513). In relation to the lack-of-fit test for the GF bread crumb grain features, all regression models yielded a highly significant lack-of-fit (
p < 0.0001).
In relation to CV, the lowest CV value was for VFR (8.92%), while the highest one was for MCA (31.2%). Moreover, only VFR (8.92%) showed a CV value below 10%, whereas CDE (21.1%) and MCA had values higher than 10% of CV. Crumb grain features of CDE, VFR, COM, and ARA had lower PRESS values than MCA and UNI. For each of the GF bread crumb grain features, the model’s F-test and the linear mixture terms were highly significant (
p < 0.0001, in
Table 7). All the crumb grain features were affected by the interaction XG × GG (
p < 0.05). The interaction term XG × HPMC was significant (
p < 0.05) for CDE, UNI, COM, and ARA; while the interaction term GG × HPMC was significant (
p < 0.05) for CDE, UNI, and VFR.
The response surfaces suggest that crumb grain of higher mean cell area (i.e., more open crumb grains) can be obtained with a combination of a low XG dose (0.04%), a high GG dose (0.60%), and a high-intermediate HPMC dose (3.36%;
Figure 5). Higher cell density values (i.e., denser crumb appearance) were produced with combinations of a high XG dose (0.60%) and intermediate doses of GG (0.36%) and HPMC (3.04%). Higher crumb void fraction values (i.e., greater porosity structure) were obtained with a combination of a high GG dose (0.60%), a high-intermediate HPMC dose (3.36%) and a low XG dose (0.04%).
Bourekoua et al. [
3] obtained a denser crumb appearance with a lower value of MCA (1.87 mm
2). Sciarini et al. [
35], formulating GF breads with hydrocolloids from different sources, obtained lower values for crumb UNI (ranged from 1.42 to 2.07), comparable values for MCA (2.73–8.06 mm
2), and higher values for CDE (0.89–1.67 cells/mm
2), in a matrix of rice flour (45%), cassava starch (45%), soy flour (10%), WC (75%), and hydrocolloid (0.5%). As found in this study, they also concluded that GF breads often produce a dense crumb structure with thick cell walls; hence, the presence of larger cells producing a spongier crumb is not easily obtained in GF breads.
In this specific topic, considering that from a consumer viewpoint, bread loaves of more open crumbs and greater cell size (
Figure 4; formulations 3, 8, or 9) are preferred than compact, denser and closer crumbs of smaller pores (
Figure 4; formulations 5, 10, or 11), blends of a low XG dose (0.04%), a high GG dose (0.60%), and a high-intermediate HPMC dose (3.36%) would be suitable to achieve GF bread crumbs of lower value of CDE (0.10 cells/ mm
2), and higher values of MCA (4.17 mm
2) and VFR (0.45).
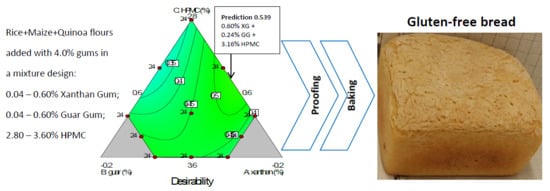
3.5. Optimization of the Gum Mixtures on Main GF Bread Quality Parameters
The quality requirements for bread production can be set from the producer and/or the consumer perspective. One of the most important parameters in GF breadmaking, known to strongly influence consumer’s choice, is the loaf specific volume, as larger loaves are perceived as more appealing. According to Dapčević-Hadnađev et al. [
36], the quality characteristics that determine the choice of bread by the consumer are those related to its appearance, such as the size (volume) of the bread, color (crust color), bread shape, and freshness (related with the crumb softness), which frequently is checked by pressing the bread. As stated by Živančev, et al. [
37], the crust color of bread is a very important parameter for consumers because the acceptability of the product strongly depends on a golden brown color [
38]. Furthermore, bread crumb grain, perceived by the consumers as degree of porosity, is another important quality characteristic which indicates softness and foamy crumb structure [
38]. Therefore, with basis on the information above, to determine the optimal hydrocolloids mixture, the optimization was carried out by maximizing the bread specific volume (mL/g), and at the same time minimizing the crust luminosity, crumb hardness (g) and mean cell density (cells/mm
2). The bread quality features to optimize were assigned the same relative importance. The optimal formulation of 3.16% HPMC, 0.60% GG, and 0.24% XG produced a desirability of 0.54. This optimized mixture of gums would produce in the final bread product, the following estimated values for specific volume: 2.57 mL/g; crust L*: 67.0; crumb hardness: 2082 g; and mean cell density: 0.142 cells/mm
2. The maximum prediction point indicated in
Figure 6 was determined by numerical optimization of the polynomial models, by setting the factors goals ‘within the range’ and the variable responses goal to ‘maximize’ or ‘minimize’.
In relation to the hydrocolloids’ interaction effect on the GF bread properties, it was found that XG × GG interacted to increase batter firmness, bread specific volume, baking loss, crumb aw, crust lightness, crumb hardness, adhesiveness, mean cell density, and cell size uniformity; while decreasing batter stickiness, crumb cohesiveness, resilience, mean cell area, and void fraction. XG interacted with HPMC to increase batter firmness, bread crumb hardness, and adhesiveness; and to reduce batter stickiness, cohesiveness, resilience, and mean cell density, cell size uniformity, and void fraction. Furthermore, GG also interacted with HPMC to increase batter firmness, bread specific volume, crumb aw, crust and crumb lightness, crumb hardness, adhesiveness, mean cell density, and cell size uniformity; and to decrease batter stickiness, bread baking loss, cohesiveness, and resilience. The three-way interaction XG × GG × HPMC was not assessed in this study.