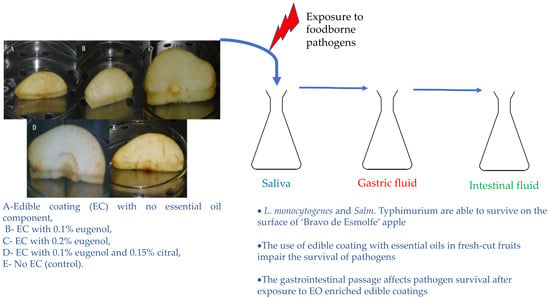
Edible Coatings Enriched with Essential Oils on Apples Impair the Survival of Bacterial Pathogens through a Simulated Gastrointestinal System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Essential Oils Components
2.3. Fruit
2.4. Organic Acid Content
2.5. Determination of pH
2.6. Edible Coatings
2.7. Survival of Bacteria on the Surface of Apples
2.8. Exposure to Simulated Gastrointestinal Fluids
2.9. Statistical Analyses
3. Results
3.1. Organic Acids, Sugar, and pH
3.2. Survival of Foodborne Pathogens on the Surface of ‘Bravo de Esmolfe’ Apples
3.3. The Effect of Edible Coatings on the Survival of the Foodborne Pathogens in Fresh-Cut Apples
3.4. The Impact of Edible Coatings on the Ability of Foodborne Pathogens to Overcome a Simulated Gastrointestinal Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on the Risk Posed by Pathogens in Food of Non-Animal Origin. Part 1 (Outbreak Data Analysis and Risk Ranking of Food/Pathogen Combinations). Annex EFSA J. 2013, 11, 3025. [Google Scholar] [CrossRef]
- Painter, J.A.; Hoekstra, R.M.; Ayers, T.; Tauxe, R.V.; Branden, C.R. Attribution of Foodborne Illnesses, Hospitalizations, and Deaths to Food Commodities by Using Outbreak Data, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 407. [Google Scholar] [CrossRef]
- Center Disease Control. Multistate Outbreak of Listeriosis Linked to Commercially Produced, Prepackaged Caramel Apples Made from Bidart Bros. Apples (Final Update); Centers for Disease Control and Prevention: Atlanta, GA, USA, 2015. [Google Scholar]
- Aune, D.; Giovannucci, E.B.P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.T.S. Fruit and Vegetable Intake and the Risk of Cardiovascular Disease, Total Cancer and Allcause Mortality—A Systematic Review and Doseresponse Meta-Analysis of Prospective Studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Trapl, E.S.; Smith, S.; Joshi, K.; Osborne, A.; Matos, A.T.; Bolen, S. Dietary Impact of Produce Prescriptions for Patients with Hypertension. Prev. Chronic Dis. 2018, 15, 180301. [Google Scholar] [CrossRef] [PubMed]
- WHO. Increasing Fruit and Vegetable Consumption to Reduce the Risk of Noncommunicable Diseases. e-Library of Evidence for Nutrition Actions (ELENA). e-Library Evid. Nutr. Actions 2018. Available online: https://www.who.int/elena/titles/fruit_vegetables_ncds/en/.
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards); Ricci, A.A.A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Herman, L.K.K.; Nørrung, B.; Robertson, L.; et al. Scientific Opinion on the Listeria Monocytogenes Contamination of Ready-to-Eat Foods and the Risk for Human Health in the EU. EFSA J. 2018, 16, 5134. [Google Scholar]
- Feng, Y.; Li, G.; Lv, X.; Xu, Y.; Wu, Q.; Shi, C.; Li, Q.; Yang, B.; Wang, X.; Xi, M.; et al. Prevalence, Distribution, and Diversity of Escherichia Coli, Staphylococcus Aureus, and Salmonella in Kiwifruit Orchards and Processing Plants. Foodborne Pathog. Dis. 2014, 11, 782–790. [Google Scholar] [CrossRef]
- Gautam, D.; Dobhal, S.; Payton, M.E.; Fletcher, J.; Ma, L.M. Surface Survival and Internalization of Salmonella through Natural Cracks on Developing Cantaloupe Fruits, Alone or in the Presence of the Melon Wilt Pathogen Erwinia Tracheiphila. PLoS ONE 2014, 9, e105248. [Google Scholar] [CrossRef]
- Blackburn, B.G.; Mazurek, J.M.; Hlavsa, M.; Park, J.; Tillapaw, M.; Parrish, M.K.; Salehi, E.; Franks, W.; Koch, E.; Smith, F.; et al. Cryptosporidiosis Associated with Ozonated Apple Cider. Emerg. Infect. Dis. 2006, 12, 684–686. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Outbreaks of Escherichia Coli O157:H7 Infection and Cryptosporidiosis Associated with Drinking Unpasteurized Apple Cider—Connecticut and New York, October 1996. Annex MMWR 1997, 46, 4–8. [Google Scholar]
- Canadian Food Inspection Agency (CFIA). Food Recall Warning—Unpasteurized Apple Cider Processed by Rolling Acres Cider Mill Recalled due to E. coli O157:H7. Available online: http://www.inspection.gc.ca/about-the-cfia/newsroom/food-recall-warnings/complete-listing/2014-10-30/eng/1414720185030/1414720197088 (accessed on 2 February 2015).
- Faleiro, M.L. Response of Foodborne Bacteria to Acid Shock. In Stress Response of Foodborne Pathogens; Nova Press: New York, NY, USA, 2012; pp. 35–70. [Google Scholar]
- Antunes, M.; Gago, C.; Cavaco, A.; Miguel, M.G. Edible Coatings Enriched with Essential Oils and Their Compounds for Fresh and Fresh-Cut Fruit. Recent Patents Food Nutr. Agric. 2012, 4, 114–122. [Google Scholar] [CrossRef]
- Kalia, A.; Parshad, V.R. Novel Trends to Revolutionize Preservation and Packaging of Fruits/Fruit Products: Microbiological and Nanotechnological Perspectives. Crit. Rev. Food Sci. Nutr. 2013, 55, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Alegre, I.; Abadias, M.; Anguera, M.; Oliveira, M.; Viñas, I. Factors Affecting Growth of Foodborne Pathogens on Minimally Processed Apples. Food Microbiol. 2010, 27, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rodriguez, F.; Begum, M.; Johannssen, G.S. Study of the Cross-Contamination and Survival of Salmonella in Fresh Apples. Int. J. Food Microbiol. 2014, 184, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Raybaudi-Massilia, R.M.; Rojas-Graü, M.A.; Mosqueda-Melgar, J.; Martín-Belloso, O. Comparative Study on Essential Oils Incorporated into an Alginate-Based Edible Coating to Assure the Safety and Quality of Fresh-Cut Fuji Apples. J. Food Prot. 2008, 71, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M. Antioxidant Activity of Medicinal and Aromatic Plants. A Review. Flavour Fragr. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The Effect of Alginate-Based Edible Coatings Enriched with Essential Oils Constituents on Arbutus Unedo L. Fresh Fruit Storage. Postharvest Biol. Technol. 2015, 100. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. Edible Coatings Enriched with Essential Oils for Extending the Shelf-Life of “Bravo de Esmolfe” Fresh-Cut Apples. Int. J. Food Sci. Technol. 2016, 51. [Google Scholar] [CrossRef]
- Moldão-Martins, M.; Beirão-da-Costa, S.M.; Beirão-da-Costa, M.L. The Effects of Edible Coatings on Postharvest Quality of the “Bravo de Esmolfe” Apple. Eur. Food Res. Technol. 2003, 217, 325–328. [Google Scholar] [CrossRef]
- Crespo, P.; Bordonaba, J.G.; Terry, L.A.; Carlen, C. Characterization of Major Taste and Health-Related Compounds of Four Strawberry Genotypes Grown at Different Swiss Production Sites. Food Chem. 2010, 122, 16–24. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L.; Terry, L.A.; Landahl, S.; Cronje, P.J.R.; Nieuwoudt, H.H.; Hanssens, A.; Saeys, W.; Nicolaï, B.M. Evaluation of Fourier Transform-NIR Spectroscopy for Integrated External and Internal Quality Assessment of Valencia Oranges. J. Food Compos. Anal. 2013, 31, 144–154. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Raybaudi-Massilia, R.M.; Soliva-Fortuny, R.C.; Avena-Bustillos, R.J.; McHugh, T.H.; Martín-Belloso, O. Apple Puree-Alginate Edible Coating as Carrier of Antimicrobial Agents to Prolong Shelf-Life of Fresh-Cut Apples. Postharvest Biol. Technol. 2007, 45, 254–264. [Google Scholar] [CrossRef]
- Versantvoort, C.H.M.; Oomen, A.G.; Kamp, E.; Rompelberg, C.J.M.; Sips, A.J. Applicability of an in Vitro Digestion Model in Assessing the Bioaccessibility of Mycotoxins from Food. Food Chem. Toxicol. 2005, 43, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Melo, J.; Schrama, D.; Hussey, S.; Andrew, P.W.; Faleiro, M.L. Listeria Monocytogenes Dairy Isolates Show a Different Proteome Response to Sequential Exposure to Gastric and Intestinal Fluids. Int. J. Food Microbiol. 2013, 163. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Chen, J.; Zheng, H.; Fang, T.; Ogutu, C.; Li, S.; Han, Y.; Wu, B. Comparative Assessment of Sugar and Malic Acid Composition in Cultivated and Wild Apples. Food Chem. 2015, 172, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, P.; Cheng, L. Developmental Changes of Carbohydrates, Organic Acids, Amino Acids, and Phenolic Compounds in ‘Honeycrisp’ Apple Flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- Tian, J.Q.; Bae, Y.M.; Lee, S.Y. Survival of Foodborne Pathogens at Different Relative Humidities and Temperatures and the Effect of Sanitizers on Apples with Different Surface Conditions. Food Microbiol. 2013, 35, 21–26. [Google Scholar] [CrossRef]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of Chitosan-Beeswax Edible Coatings on the Quality of Fresh Strawberries (Fragaria Ananassa Cv Camarosa) under Commercial Storage Conditions. LWT—Food Sci. Technol. 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Reis, S.F.A.R.; Rocha, S.M.; Barros, A.S.; Delgadillo, I.; Coimbra, M.A. Establishment of the Volatile Profile of ‘Bravo de Esmolfe’ Apple Variety and Identification of Varietal Markers. Food Chem. 2009, 113, 513–521. [Google Scholar] [CrossRef]
- Faleiro, M.L. The Mode of Antibacterial Action of Essential Oils. In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; pp. 1143–1156. [Google Scholar]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an Essential Oil of Clove) Acts as an Antibacterial Agent against Salmonella Typhi by Disrupting the Cellular Membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Somolinos, M.; García, D.; Condón, S.; Mackey, B.; Pagan, R. Inactivation of Escherichia Coli by Citral. J. Appl. Microbiol. 2009, 108, 1928–1939. [Google Scholar] [CrossRef] [PubMed]
- Chueca, B.; Pagán, R.; Garcia-Gonzalo, D. Oxygenated Monoterpenes Citral and Carvacrol Cause Oxidative Damage in Escherichia Coli without the Involvement of Tricarboxylic Acid Cycle and Fenton Reaction. Int. J. Food Microbiol. 2014, 189, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Siroli, L.; Patrignani, F.; Gardini, F.; Lanciotti, R. Effects of Sub-Lethal Concentrations of Thyme and Oregano Essential Oils, Carvacrol, Thymol, Citral and Trans-2-Hexenal on Membrane Fatty Acid Composition and Volatile Molecule Profile of Listeria Monocytogenes, Escherichia Coli and Salmonella Enteritidis. Food Chem. 2014, 182, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Sobrino-Lopez, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of Malic Acid and Other Quality Stabilizing Compounds to Assure the Safety of Fresh-Cut “Fuji” Apples by Inactivation of Listeria Monocytogenes, Salmonella Enteritidis and Escherichia Coli O157:H7. J. Food Saf. 2009, 29, 236–252. [Google Scholar] [CrossRef]
- Colás-Medà, P.; Abadias, M.; Oliveira, M.; Usall, J.; Viñas, I. Influence of Fruit Matrix and Storage Temperature on the Survival of Listeria Monocytogenes in a Gastrointestinal Simulation. Food Control 2017, 73, 1045–1052. [Google Scholar] [CrossRef]
- Álvarez-Ordóñez, A.; Begley, M.; Prieto, M.; Messens, W.; López, M.; Bernardo, A.; Hill, C. Salmonella Spp. Survival Strategies within the Host Gastrointestinal Tract. Microbiol. (United Kingdom) 2011, 157, 3268–3281. [Google Scholar]



| Fruit | Oxalic Acid | Malic Acid | Quinic Acid | Fructose | Glucose | Sucrose | pH |
|---|---|---|---|---|---|---|---|
| “Bravo de Esmolfe” | 1.87 ± 0.42 a | 1.71± 0.26 a | 1.16 ± 0.22 a | 17.61±1.13 a | 6.14±0.36 a | 4.93±1.25 a | 4.25 ± 0.06 b |
| “Golden Delicious” | 1.57 ± 0.21 a | 1.25 ± 0.36 a | 0.88 ± 0.22 a | 15.71±0.02 b | 6.81±0.30 a | 3.67±0.05 a | 4.97 ± 0.04 a |
| Time (Days) | Survival (%) | |
|---|---|---|
| L. monocytogenes 12.04 | Salm. Typhimurium ATCC 14028 | |
| 0 | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| 1 | 81.27 ± 10.30 b | 56.38 ± 8.90 b |
| 2 | 75.73 ± 4.87 b | 46.76 ± 5.11 c |
| 3 | 74.02 ± 5.34 b | 54.74 ± 6.07 b |
| 7 | 73.64 ± 3.48 b | NR |
| Fruit | Time (h) | L. monocytogenes 12.04 (% Survival) | Salm. Typhimurium ATCC 14028 (% Survival) | ||||
|---|---|---|---|---|---|---|---|
| Control | ALG (2%) with 0.2% EUG | ALG (2%) with 0.1% EUG and 0.15 % CIT | Control | ALG (2%) with 0.2% EUG | ALG (2%) with 0.1% EUG and 0.15 % CIT | ||
| ‘Bravo de Esmolfe’ | 0 | 100.0 ± 0.0 aA | 100.0 ± 0.0 aA | 100.0 ± 0.0 aA | 100.0 ± 0.0 aA | 100.0 ± 0.0 aA | 100.0 ± 0.0 aA |
| 24 | 62.50 ± 2.34 aD | 56.24 ± 8.28 bC | 57.94 ± 2.63 b,cD | 75.83 ± 12.78 aB | 56.56 ± 3.43 bC | 55.64 ± 5.63 bC | |
| 48 | 60.68 ± 5.21 aD | 58.56 ± 8.19 bC | 59.72 ± 3.93 bD | 61.59 ± 4.13 aC | 52.36 ± 3.83 bC | 50.94 ± 3.64 bC | |
| 72 | 62.58 ± 4.56 aD | 58.37 ± 3.31 bC | 55.55 ± 3.75 cD | 60.48 ± 5.67 aC | 55.60 ± 4.89 bC | 52.70 ± 5.80 bC | |
| ‘Golden Delicious’ | 0 | 100.0 ± 0.0 aA | 100.0 ± 0.00 aA | 100.0 ± 0.0 aA | 100.0 ± 0.0 aA | 100.0 ± 0.0 aA | 100.0 ± 0.0 aA |
| 24 | 72.17 ± 5.89 aB,C | 67.38 ± 4.78 bB | 70.74 ± 5.45 aB,C | 75.13 ± 4.94 aB | 67.45 ± 5.35 b,cB | 66.68 ± 8.03 cB | |
| 48 | 70.55 ± 5.57 aC | 67.38 ± 7.21 aB | 67.77 ± 5.38 aC | 71.15 ± 7.62 aB | 65.13 ± 6.84 bB | 63.86 ± 4.58 bB | |
| 72 | 76.07 ± 4.48 aB | 74.52 ± 6.44 a,bB | 73.67 ± 4.94 a,bB | 72.85 ± 4.40 aB | 68.54 ± 3.96 aB | 67.21 ± 9.40 aB | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, A.I.; Guerreiro, A.; Antunes, M.D.; Miguel, M.d.G.; Faleiro, M.L. Edible Coatings Enriched with Essential Oils on Apples Impair the Survival of Bacterial Pathogens through a Simulated Gastrointestinal System. Foods 2019, 8, 57. https://doi.org/10.3390/foods8020057
Vieira AI, Guerreiro A, Antunes MD, Miguel MdG, Faleiro ML. Edible Coatings Enriched with Essential Oils on Apples Impair the Survival of Bacterial Pathogens through a Simulated Gastrointestinal System. Foods. 2019; 8(2):57. https://doi.org/10.3390/foods8020057
Chicago/Turabian StyleVieira, Ana Isabel, Adriana Guerreiro, Maria Dulce Antunes, Maria da Graça Miguel, and Maria Leonor Faleiro. 2019. "Edible Coatings Enriched with Essential Oils on Apples Impair the Survival of Bacterial Pathogens through a Simulated Gastrointestinal System" Foods 8, no. 2: 57. https://doi.org/10.3390/foods8020057