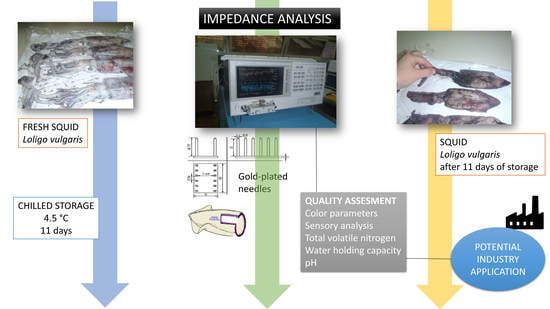
Utilizing Impedance for Quality Assessment of European Squid (Loligo Vulgaris) during Chilled Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Squid Samples
2.2. Electrical Impedance Spectroscopy Analysis
2.3. Measurement of Color Parameters
2.4. Determination of Total Volatile Base Nitrogen, pH and Water Holding Capacity
2.5. Sensory Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Electrical Measurements
3.2. Measurements of Color Parameters
3.3. Determination of Total Volatile Nitrogen Base, pH, and Water Holding Capacity
3.4. Correlations Between Measured Parameters
3.5. Sensory Evaluation of the Raw Squid
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ottinger, M.; Clauss, K.; Kuenzer, C. Aquaculture: Relevance, distribution, impacts and spatial assessments—A review. Ocean Coast. Manag. 2016, 119, 244–266. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S. Natural preservatives for extending the shelf-life of seafood: A revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1595–1612. [Google Scholar] [CrossRef]
- Atayeter, S.; Ercoşkun, H. Chemical composition of European squid and effects of different frozen storage temperatures on oxidative stability and fatty acid composition. J. Food Sci. Technol. 2010, 48, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Vaz-Pires, P.; Seixas, P.; Mota, M.; Lapa-Guimarães, J.; Pickova, J.; Lindo, A.; Silva, T. Sensory, microbiological, physical and chemical properties of cuttlefish (Sepia officinalis) and broadtail shortfin squid (Illex coindetii) stored in ice. LWT—Food Sci. Technol. 2008, 41, 1655–1664. [Google Scholar] [CrossRef]
- Vaz-Pires, P.; Seixas, P. Development of new quality index method (QIM) schemes for cuttlefish (Sepia officinalis) and broadtail shortfin squid (Illex coindetii). Food Control 2006, 17, 942–949. [Google Scholar] [CrossRef]
- Wu, T.H.; Bechtel, P.J. Ammonia, dimethylamine, trimethylamine, and trimethylamine oxide from raw and processed fish by-products. J. Aquat. Food Prod. Technol. 2008, 17, 27–38. [Google Scholar] [CrossRef]
- Amegovu, A.K.; Sserunjogi, M.L.; Ogwok, P.; Makokha, V. Nucleotide degradation products, total volatile basic nitrogen, sensory and microbiological quality of nile perch (Lates niloticus) fillets under chill storage. J. Microbiol. Biotechnol. Food Sci. 2012, 2, 653–666. [Google Scholar]
- Altissimi, S.; Mercuri, M.L.; Framboas, M.; Tommasino, M.; Pelli, S.; Benedetti, F.; Di Bella, S.; Haouet, N. Indicators of protein spoilage in fresh and defrosted crustaceans and cephalopods stored in domestic condition. Ital. J. Food Saf. 2018, 6, 6921. [Google Scholar] [CrossRef]
- LeBlanc, R.J.; Gill, T.A. Ammonia as an objective quality index in squid. Can. Inst. Food Sci. Technol. J. 1984, 17, 195–201. [Google Scholar] [CrossRef]
- Castro, P.; Padro, J.C.P.; Cansino, M.J.C.; Velazquez, E.S.; Larriva, R.M.D. Total volatile base nitrogen and its use to assess freshness in European sea bass stored in ice. Food Control 2006, 17, 245–248. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Quality and Quality Changes in Fresh Fish; United Nations: Rome, Italy, 1995. [Google Scholar]
- Huang, S.C.; Chiou, T.H.; Marshall, J.; Reinhard, J. Spectral sensitivities and color signals in a polymorphic damselfly. PLoS ONE 2014, 9, e87972. [Google Scholar] [CrossRef] [PubMed]
- Zaragozá, P.; Fuentes, A.; Ruiz-Rico, M.; Vivancos, J.-L.; Fernández-Segovia, I.; Ros-Lis, J.V.; Barat, J.M.; Martínez-Máñez, R. Development of a colorimetric sensor array for squid spoilage assessment. Food Chem. 2015, 175, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, E.; Okamoto, M.; Ozawa, A.; Fujita, T. Characterization of common squid using several freshness indicators. J. Food Sci. 1991, 56, 161–163. [Google Scholar] [CrossRef]
- Zavadlav, S.; Janči, T.; Lacković, I.; Karlović, S.; Rogulj, I.; Vidaček, S. Assessment of storage shelf life of European squid (Cephalopod: Loliginidae, Loligo vulgaris) by bioelectrical impedance measurements. J. Food Eng. 2016, 184, 44–52. [Google Scholar] [CrossRef]
- Kagawa, M.; Matsumoto, M.; Yoneda, C.; Mitsuhashi, T.; Hatae, K. Changes in meat texture of three varieties of squid in the early of stage of cold storage. Fish. Sci. 2002, 68, 783–792. [Google Scholar] [CrossRef]
- Hurtado, J.L.; Borderías, J.; Montero, P.; An, H. Characterization of proteolytic activity in octopus (Octopus vulgaris) arm muscle. J. Food Biochem. 1990, 23, 469–483. [Google Scholar] [CrossRef]
- Hartman, K.J.; Margraf, F.J.; Hafs, A.W.; Cox, M.K. Bioelectrical impedance analysis: A new tool for assessing fish condition. Fisheries 2015, 40, 590–600. [Google Scholar] [CrossRef]
- Aberg, P. Electronic Biopsis—Applications and Data Analysis; Karolinska Instituten: Stockholm, Sweden, 2002. [Google Scholar]
- Masot, R.; Alcañiz, M.; Fuentes, A.; Schmidt, F.C.; Barat, J.M.; Gil, L.; Baigts, D.; Martínez-Máñez, R.; Soto, J. Design of a low-cost non-destructive system for punctual measurements of salt levels in food products using impedance spectroscopy. Sens. Actuators A Phys. 2010, 158, 217–223. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, H.; Luo, Y. Study on the electric conduction properties of fresh and frozen-thawed grass carp (Ctenopharyngodon idellus) and tilapia (Oreochromis niloticus). Int. J. Food Sci. Technol. 2010, 45, 2560–2564. [Google Scholar] [CrossRef]
- Rizo, A.; Fuentes, A.; Fernández-Segovia, I.; Masot, R.; Alcañiz, M.; Barat, J.M. Development of a new salmon salting-smoking method and process monitoring by impedance spectroscopy. LWT—Food Sci. Technol. 2013, 51, 218–224. [Google Scholar] [CrossRef]
- Pérez-Esteve, E.; Fuentes, A.; Grau, R.; Fernández-Segovia, I.; Masot, R.; Alcañiz, M.; Barat, J.M. Use of impedance spectroscopy for predicting freshness of sea bream (Sparus aurata). Food Control 2014, 35, 360–365. [Google Scholar] [CrossRef]
- Schwan, H.P. Dielectric properties of biological tissue and biophysical mechanisms of electromagnetic-field interaction. In Biological Effects of Nonionizing Radiation; Illinge, K.H., Ed.; American Chemical Society: Houston, TX, USA, 1981; pp. 109–131. [Google Scholar]
- Grimnes, S.; Martinsen, Ø.G. Sources of error in tetrapolar impedance measurements on biomaterials and other ionic conductors. J. Phys. D Appl. Phys. 2007, 40, 9–14. [Google Scholar] [CrossRef]
- Mazzeo, B.A.; Flewitt, A.J. Two- and four-electrode, wide-bandwidth, dielectric spectrometer for conductive liquids: Theory, limitations, and experiment. J. Appl. Phys. 2007, 102, 6–11. [Google Scholar] [CrossRef]
- Ishai, P.B.; Talary, M.S.; Caduff, A.; Levy, E.; Feldman, Y. Electrode polarization in dielectric measurements: A review. Meas. Sci. Technol. 2013, 24, 1–21. [Google Scholar] [CrossRef]
- Ćurić, T.; Marušić Radovčić, N.; Janči, T.; Lacković, I.; Vidaček, S. Salt and moisture content determination of fish by bioelectrical impedance and a needle-type multi-electrode array. Int. J. Food Prop. 2016, 20, 2477–2486. [Google Scholar] [CrossRef]
- Antonacopoulos, N.; Vyncke, W. Determination of volatile basic nitrogen in fish: A third collaborative study by the West European Fish Technologists’ Association (WEFTA). Z. Lebensm. Unters. Forsch. 1989, 189, 309–316. [Google Scholar] [CrossRef]
- Benjakul, S.; Morrissey, M.T.; Seymour, T.A.; An, H. Recovery of proteinase from Pacific whiting surimi wash water. J. Food Biochem. 1997, 21, 431–443. [Google Scholar] [CrossRef]
- Grau, R.; Hamm, R. A simple method for the determination of water binding in muscles. Naturwissenschaften 1953, 40, 29–30. [Google Scholar] [CrossRef]
- Howgate, P.; Johnston, A.; Whittle, K.J. Multilingual Guide to EC Freshness Grades for Fishery Products; Torry Research Station, Food Safety Directorate, Ministry of Agriculture, Fisheries and Food: Aberdeen, UK, 1992; Volume 32. [Google Scholar]
- Vidaček, S.; Medić, H.; Botka-Petrak, K.; Nežak, J.; Petrak, T. Bioelectrical impedance analysis of frozen sea bass (Dicentrarchus labrax). J. Food Eng. 2008, 88, 263–271. [Google Scholar] [CrossRef]
- Vidaček, S.; Janči, T.; Marušić, N.; Petrak, T.; Vnučec, T.; Medić, H. Effects of freezing on impedance of rainbow trout (Oncorhynchus mykiss). Croat. J. Fish. 2011, 60, 39–50. [Google Scholar]
- Vidaček, S.; Janči, T.; Brdek, Z.; Udovičić, D.; Marušić, N.; Medić, H.; Petrak, T.; Lacković, I. Differencing sea bass (Dicentrarchus labrax) fillets frozen in different conditions by impedance measurements. Int. J. Food Sci. Technol. 2012, 47, 1757–1764. [Google Scholar] [CrossRef]
- Vidaček, S.; Medić, H.; Marušić, N.; Tonković, S.; Petrak, T. Influence of different freezing regimes on bioelectrical properties of atlantic chub mackerel (Scomber Colias). J. Food Process Eng. 2012, 35, 735–741. [Google Scholar] [CrossRef]
- Martinsen, Ø.G.; Grimnes, S.; Mirtaheri, P. Non-invasive measurements of post mortem changes in dielectric properties of haddock muscle—A pilot study. J. Food Eng. 2002, 43, 189–192. [Google Scholar] [CrossRef]
- Ryynänen, S. The electromagnetic properties of food materials: A review of the basic principles. J. Food Eng. 1995, 26, 409–429. [Google Scholar] [CrossRef]
- Sungsri-in, R.; Benjakul, S.; Kijroongrojana, K. Pink discoloration and quality changes of squid (Loligo formosana) during iced storage. LWT—Food Sci. Technol. 2011, 44, 206–213. [Google Scholar] [CrossRef]
- Lapa-Guimarães, J.; Aparecida Azevedo da Silva, M.; Eduardo de Felício, P.; Guzman, E.C. Sensory, color and phychrotrophic bacterial analyses of squid (Loligo plei) during storage in ice. LWT—Food Sci. Technol. 2002, 35, 21–29. [Google Scholar] [CrossRef]
- Melaj, M.; Sánchez-Pascua, G.; Casales, M.R.; Yeannes, M.I. Aspectos a considerar en la evaluación de la frescura del calamar (Illex argentinus). Alimentaría 1997, 35, 93–96. [Google Scholar]
- Tomac, A.; Mascheroni, R.H.; Yeannes, M.I. Modeling total volatile basic nitrogen production as a dose function in gamma irradiated refrigerated squid rings. LWT—Food Sci. Technol. 2014, 56, 533–536. [Google Scholar] [CrossRef]
- Rehbein, H.; Oehlenschläger, J. Zur Zusammensetzung der TVB-N-Fraktion (flüchtigen Basen) in sauren Extrakten und alkalischen Destillaten von Seefischfilet. Arch. Lebensm. 1982, 33, 44–48. [Google Scholar]
- La qualité et son évolution dans le poisson frais. FAO docu-ment technique sur les Pêches 348; Huss, H.H. (Ed.) FAO: Rome, Italy, 1999. [Google Scholar]
- Bekhit, A.E.-D.A.; Suwandy, V.; Carne, A.; Mohamed Ahmed, I.A.; Wang, Z. Manipulation of Meat Quality: Electrical Stimulation and Pulsed Electric Field. In Advances in Meat Processing Technology; Bekhit, A.E.-D.A., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 121–168. [Google Scholar]
- Yamanaka, H.; Shiomi, K.; Kikuchi, T. Agmatine as a potential index for freshness of common squid (Todarodes pacificus). J. Food Sci. 1987, 52, 936–938. [Google Scholar] [CrossRef]
- Lougovois, V.; Kyrana, V.R. Freshness quality and spoilage of chill-stored fish. In Food Policy, Control and Research; Riley, A.P., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2005; pp. 35–86. [Google Scholar]
- Kumar, N.; Fournier, T.; Courtois, H.; Gupta, A.K. Fabrication and characterization of shunted μ-SQUID. AIP Conf. Proc. 2014, 1591–1594. [Google Scholar] [CrossRef]



| Day of Storage | Z 5 kHz | Z 10 kHz | Z 100 kHz | Z 1 MHz |
|---|---|---|---|---|
| 1 | 541.16 ± 69.823 a | 528.83 ± 67.92 a | 494.33 ± 62.16 a | 378.29 ± 45.79 a |
| 2 | 500.75 ± 67.60 a | 486.13 ± 65.10 a | 446.53 ± 57.8 b | 306.77 ± 32.11 b |
| 3 | 398.01 ± 81.68 b | 384.72 ± 77.30 b | 350.95 ± 69.04 c | 246.15 ± 43.45 c |
| 4 | 248.96 ± 60.93 c | 240.41 ± 59.67 c | 220.72 ± 54.83 d | 170.05 ± 39.46 d |
| 5 | 227.05 ± 28.27 c | 219.74 ± 27.15 c | 203.99 ± 24.68 d | 170.18 ± 20.35 d |
| 8 | 127.41 ± 17.50 d | 123.05 ± 17.16 d | 116.74 ± 16.47 e | 108.12 ± 15.15 e |
| 9 | 122.21 ± 14.55 d | 118.19 ± 13.74 d | 112.64 ± 12.63 e | 106.99 ± 11.58 e |
| 10 | 98.22 ± 23.21 d | 95.04 ± 22.71 d | 90.98 ± 21.81 e | 87.84 ± 21.39 ef |
| 11 | 80.33 ± 20.07 d | 77.67 ± 19.51 d | 74.63 ± 18.73 e | 72.51 ± 18.39 f |
| Day of Storage | φ 5 kHz | φ 100 kHz | φ 500 kHz | φ 1 MHz | φ 5 MHz | φ 10 MHz |
|---|---|---|---|---|---|---|
| 1 | −4.34 ± 0.31 a | −5.65 ± 0.77 b | −15.1 ± 1.93 b | −21.63 ± 2.04 b | −29.5 ± 1.42 ab | −26.63 ± 1.13 a |
| 2 | −4.99 ± 0.29 ab | −7.41 ± 0.71 a | −19.39 ± 1.68 a | −26.09 ± 1.95 a | −31.23 ± 1.42 a | −27.22 ± 1.136 a |
| 3 | −5.10 ± 0.76 ab | −7.3 ± 0.91 a | −16.82 ± 1.71 a | −22.54 ± 1.96 a | −28.7 ± 2.03 b | −25.30 ± 1.73 a |
| 4 | −6.07 ± 1.45 b | −5.8 ± 0.75 b | −13.41 ± 1.84 c | −18.0 ± 2.39 c | −24.75 ± 2.33 c | −22.65 ± 1.71 b |
| 5 | −6.02 ± 0.83 b | −4.53 ± 0.48 c | −10.13 ± 1.04 d | −14.21 ± 1.29 d | −22.89 ± 1.66 c | −21.92 ± 1.52 b |
| 8 | −7.88 ± 1.31 c | −2.6 ± 0.44 d | −4.87 ± 1.01 e | −6.97 ± 1.41 e | −14.99 ± 2.08 d | −16.28 ± 1.82 c |
| 9 | −8.47 ± 0.80 cd | −2.08 ± 0.3 de | −3.16 ± 0.79 f | −4.32 ± 1.18 f | −10.43 ± 1.92 e | −12.18 ± 1.80 d |
| 10 | −9.19 ± 1.63 d | −1.76 ± 0.36 e | −2.29 ± 0.79 f | −3.07 ± 1.08 f | −8.08 ± 1.50 f | −9.55 ± 1.67 e |
| 11 | −9.55 ± 1.58 d | −1.57 ± 0.36 e | −1.88 ± 1.09 f | −2.50 ± 1.74 f | −6.53 ± 3.29 f | −8.54 ± 3.21 e |
| Day(s) of Storage | L* | a* | b* |
|---|---|---|---|
| 1 | 43.19 ± 4.44 a | 1.53 ± 0.97 a | −1.18 ± 0.98 a |
| 2 | 40.07 ± 5.07 a | 1.78 ± 1.12 ab | −1.31 ± 1.02 a |
| 3 | 35.73 ± 3.58 b | 2.22 ± 0.79 abc | −1.63 ± 0.46 a |
| 4 | 32.37 ± 3.07 bc | 2.95 ± 1.15 bcd | 1.47 ± 1.24 b |
| 5 | 31.61 ± 2.73 bc | 3.45 ± 1.13 cde | 2.35 ± 0.71 bc |
| 8 | 30.41 ± 3.06 c | 3.86 ± 0.95 de | 2.87 ± 1.19 cd |
| 9 | 29.39 ± 4.05 c | 4.62 ± 1.12 ef | 2.92 ± 0.49 cd |
| 10 | 28.97 ± 3.50 c | 5.35 ± 1.70 f | 3.63 ± 1.16 d |
| 11 | 27.80 ± 3.76 c | 5.84 ± 1.25 f | 3.95 ± 1.11 d |
| Day of Storage | TVB−N (mg N/100 g) | WHC | pH |
|---|---|---|---|
| 2 | 11.91 ± 0.25 a | 37.07 ± 4.46 a | 6.66 ± 0.08 a |
| 5 | 16.12 ± 0.12 b | 15.94 ± 0.53 b | 6.96 ± 0.21 a |
| 9 | 63.03 ± 0.18 c | 13.76 ± 0.00 c | 7.35 ± 0.15 b |
| 11 | 207.3 ± 0.09 d | 12.34 ± 0.00 c | 7.42 ± 0.01 c |
| Days | EC | ϕ ατ 5 ΜHζ | a* | b* | WHC | TVB-N | |
|---|---|---|---|---|---|---|---|
| Days | 1 | 0.956 * | 0.968 * | 0.785 * | 0.845 * | −0.824 * | 0.848 * |
| EC | 1 | 0.935 | 0.779 * | 0.875 * | −0.831 * | 0.894 * | |
| ϕ ατ 5 ΜHζ | 1 | 0.754 * | 0.810 * | −862 * | 0.768 * | ||
| a* | 1 | 0.651 * | −0.584 * | 0.860 * | |||
| b* | 1 | −0.925 * | 0.609 | ||||
| WHC | 1 | −0.546 * | |||||
| TVB-N | 1 |
| Measurements | Storage Days | Scores According to Kumar et al. [48] | Category According to EC Scheme | Groups |
|---|---|---|---|---|
| 1 | 0.5 | 10 | E | 1 |
| 2 | 2 | 10 | E | 1 |
| 3 | 3 | 10 | E | 1 |
| 4 | 4 | 9 | E | 1 |
| 5 | 5 | 8 | A | 2 |
| 6 | 8 | 7 | A | 2 |
| 7 | 9 | 6 | B | 3 |
| 8 | 10 | 5 | B | 3 |
| 9 | 11 | 4 | B | 3 |
| 10 | 12 | 1 | C | 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavadlav, S.; Lacković, I.; Bursać Kovačević, D.; Greiner, R.; Putnik, P.; Vidaček Filipec, S. Utilizing Impedance for Quality Assessment of European Squid (Loligo Vulgaris) during Chilled Storage. Foods 2019, 8, 624. https://doi.org/10.3390/foods8120624
Zavadlav S, Lacković I, Bursać Kovačević D, Greiner R, Putnik P, Vidaček Filipec S. Utilizing Impedance for Quality Assessment of European Squid (Loligo Vulgaris) during Chilled Storage. Foods. 2019; 8(12):624. https://doi.org/10.3390/foods8120624
Chicago/Turabian StyleZavadlav, Sandra, Igor Lacković, Danijela Bursać Kovačević, Ralf Greiner, Predrag Putnik, and Sanja Vidaček Filipec. 2019. "Utilizing Impedance for Quality Assessment of European Squid (Loligo Vulgaris) during Chilled Storage" Foods 8, no. 12: 624. https://doi.org/10.3390/foods8120624