Broccoli (Brassica oleracea L. var. italica) Sprouts as the Potential Food Source for Bioactive Properties: A Comprehensive Study on In Vitro Disease Models
Abstract
:1. Introduction
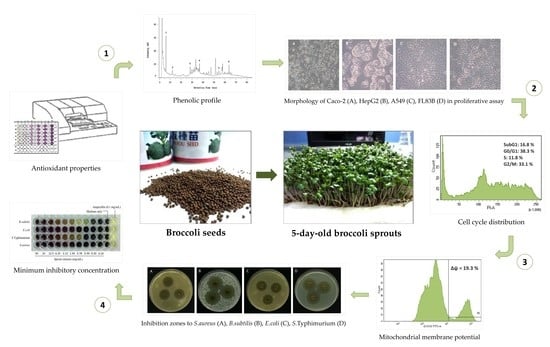
2. Materials and Methods
2.1. Plant Material and Cultivation Condition
2.2. Crude Extract Preparation
2.3. Determination of Total Phenolic Content (TPC)
2.4. Determination of Total Flavonoid Content (TFC)
2.5. Determination of Vitamin C Content
2.6. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Assay
2.7. 2,2′-Azino-Bis-3-Ethylbenzothiazoline-6-Sulphonic (ABTS) Radical Cation Decolorization Assay
2.8. Reducing Power Assay
2.9. High-Performance Liquid Chromatography (HPLC) Analysis
2.10. Cell Culture
2.11. Proliferation Assay
2.12. Cell Cycle Analysis
2.13. Measurement of Mitochondrial Membrane Potential (MMP)
2.14. Bacterial Culture
2.15. Agar Diffusion Method
2.16. Determination of the Minimum Inhibition Concentration (MIC)
2.17. Statistical Analysis
3. Results
3.1. Antioxidant Activity
3.2. Antiproliferative and Apoptotic Activities
3.3. Antibacterial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Soares, N.D.C.P.; Elias, M.D.B.; Lima Machado, C.; Trindade, B.B.; Borojevic, R.; Teodoro, A.J. Comparative Analysis of Lycopene Content from Different Tomato-Based Food Products on the Cellular Activity of Prostate Cancer Cell Lines. Foods 2019, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Severini, C.; Giuliani, R.; De Filippis, A.; Derossi, A.; De Pilli, T. Influence of different blanching methods on colour, ascorbic acid and phenolics content of broccoli. J. Food Sci. Technol. 2016, 53, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Sharma, U.; Vig, A.P.; Singh, B.; Arora, S. Free radical scavenging, antiproliferative activities and profiling of variations in the level of phytochemicals in different parts of broccoli (Brassica oleracea italica). Food Chem. 2014, 148, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Bachiega, P.; Salgado, J.M.; de Carvalho, J.E.; Ruiz, A.L.T.; Schwarz, K.; Tezotto, T.; Morzelle, M.C. Antioxidant and antiproliferative activities in different maturation stages of broccoli (Brassica oleracea Italica) biofortified with selenium. Food Chem. 2016, 190, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Pająk, P.; Socha, R.; Gałkowska, D.; Rożnowski, J.; Fortuna, T. Phenolic profile and antioxidant activity in selected seeds and sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef]
- De la Fuente, B.; López-García, G.; Mañez, V.; Alegría, A.; Barberá, R.; Cilla, A. Evaluation of the Bioaccessibility of Antioxidant Bioactive Compounds and Minerals of Four Genotypes of Brassicaceae Microgreens. Foods 2019, 8, 250. [Google Scholar] [CrossRef]
- Chaudhary, A.; Choudhary, S.; Sharma, U.; Vig, A.P.; Singh, B.; Arora, S. Purple head broccoli (Brassica oleracea L. var. italica Plenck), a functional food crop for antioxidant and anticancer potential. J. Food Sci. Technol. 2018, 55, 1806–1815. [Google Scholar] [CrossRef]
- Xuan, T.; Gangqiang, G.; Minh, T.; Quy, T.; Khanh, T. An overview of chemical profiles, antioxidant and antimicrobial activities of commercial vegetable edible oils marketed in Japan. Foods 2018, 7, 21. [Google Scholar] [CrossRef]
- Tian, M.; Xu, X.; Hu, H.; Liu, Y.; Pan, S. Optimisation of enzymatic production of sulforaphane in broccoli sprouts and their total antioxidant activity at different growth and storage days. J. Food Sci. Technol. 2017, 54, 209–218. [Google Scholar] [CrossRef]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D. UVA, UVB light, and methyl jasmonate, alone or combined, redirect the biosynthesis of glucosinolates, phenolics, carotenoids, and chlorophylls in broccoli sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef]
- Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Panjwani, A.A.; Liu, H.; Cornblatt, G.; Cornblatt, B.S.; Ownby, S.L.; Fuchs, E.; Holtzclaw, W.D. Bioavailability of Sulforaphane Following Ingestion of Glucoraphanin-Rich Broccoli Sprout and Seed Extracts with Active Myrosinase: A Pilot Study of the Effects of Proton Pump Inhibitor Administration. Nutrients 2019, 11, 1489. [Google Scholar] [CrossRef] [PubMed]
- Ares, A.M.; Nozal, M.J.; Bernal, J. Extraction, chemical characterization and biological activity determination of broccoli health promoting compounds. J. Chromatogr. A 2013, 1313, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Paśko, P.; Tyszka-Czochara, M.; Galanty, A.; Gdula-Argasińska, J.; Żmudzki, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S. Comparative Study of Predominant Phytochemical Compounds and Proapoptotic Potential of Broccoli Sprouts and Florets. Plant Foods Hum. Nutr. 2018, 73, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawlik-Dziki, U.; Jeżyna, M.; Świeca, M.; Dziki, D.; Baraniak, B.; Czyż, J. Effect of bioaccessibility of phenolic compounds on in vitro anticancer activity of broccoli sprouts. Food Res. Int. 2012, 49, 469–476. [Google Scholar] [CrossRef]
- Sibi, G.; Shukla, A.; Dhananjaya, K.; Ravikumar, K.; Mallesha, H. In vitro antibacterial activities of Broccoli (Brassica oleracea L. var italica) against food borne bacteria. J. Appl. Pharm. Sci. 2013, 3. [Google Scholar] [CrossRef]
- Pacheco-Cano, R.; Salcedo-Hernández, R.; López-Meza, J.; Bideshi, D.; Barboza-Corona, J. Antimicrobial activity of broccoli (Brassica oleracea var. italica) cultivar Avenger against pathogenic bacteria, phytopathogenic filamentous fungi and yeast. J. Appl. Microbiol. 2018, 124, 126–135. [Google Scholar] [CrossRef]
- Sanz-Puig, M.; Pina-Pérez, M.C.; Criado, M.N.; Rodrigo, D.; Martínez-López, A. Antimicrobial potential of cauliflower, broccoli, and okara byproducts against foodborne bacteria. Foodborne Pathog. Dis. 2015, 12, 39–46. [Google Scholar] [CrossRef]
- Baenas, N.; Moreno, D.A.; García-Viguera, C. Selecting sprouts of Brassicaceae for optimum phytochemical composition. J. Agric. Food Chem. 2012, 60, 11409–11420. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S. A comparative study on the polyphenolic content, antibacterial activity and antioxidant capacity of different solvent extracts of Brassica oleracea vegetables. Int. J. Food Sci. Technol. 2012, 47, 223–231. [Google Scholar] [CrossRef]
- Ibrahim, F.Y.; EL-Khateeb, A.Y.; Mohamed, A.H. Rhus and Safflower Extracts as Potential Novel Food Antioxidant, Anticancer, and Antimicrobial Agents Using Nanotechnology. Foods 2019, 8, 139. [Google Scholar] [CrossRef]
- Porter, Y. Antioxidant properties of green broccoli and purple-sprouting broccoli under different cooking conditions. Biosci. Horiz. Int. J. Stud. Res. 2012, 5. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, M.D.C.; Moreno, D.A.; Carvajal, M.; García-Viguera, C.; Martínez-Ballesta, M.D.C. Natural antioxidants in purple sprouting broccoli under Mediterranean climate. J. Food Sci. 2012, 77, C1058–C1063. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.; Guimarães, D.; Berniz, C.; Abreu, J.; Rocha, A.; Moura, R.; Resende, A.; Teodoro, A. Açai (Euterpe oleracea Mart.) Seed Extract Induces Cell Cycle Arrest and Apoptosis in Human Lung Carcinoma Cells. Foods 2018, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Ma, Y.; Yan, D. Antioxidant and antimicrobial properties of water soluble polysaccharide from Arachis hypogaea seeds. J. Food Sci. Technol. 2014, 51, 2839–2844. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Gao, Y.; Lai, P.-X. Chemical Composition, Antioxidant, Antimicrobial and Cytotoxic Activities of Essential Oil from Premna microphylla Turczaninow. Molecules 2017, 22, 381. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, B.S.; Park, H.R.; Yu, S.B.; Kang, H.M.; Kim, I.R. XIAP inhibitor embelin induces autophagic and apoptotic cell death in human oral squamous cell carcinoma cells. Environ. Toxicol. 2017, 32, 2371–2378. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Xu, J.; Liu, X.; Xia, X.; Li, N.; Bi, X. Shikonin induces apoptosis in the human gastric cancer cells HGC-27 through mitochondria-mediated pathway. Pharmacogn. Mag. 2015, 11, 250. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Silva, A.C.O.; Santana, E.F.; Saraiva, A.M.; Coutinho, F.N.; Castro, R.H.A.; Pisciottano, M.N.C.; Amorim, E.L.C.; Albuquerque, U.P. Which approach is more effective in the selection of plants with antimicrobial activity? Evid.-Based Complement. Altern. Med. 2013, 2013, 308980. [Google Scholar] [CrossRef]
- Silva, A.P.; Nascimento da Silva, L.C.; da Fonseca, M.; Caíque, S.; de Araújo, J.M.; Correia, M.T.; Cavalcanti, M.d.S.; Lima, V.L. Antimicrobial Activity and Phytochemical Analysis of Organic Extracts from Cleome spinosa Jaqc. Front. Microbiol. 2016, 7, 963. [Google Scholar] [CrossRef] [PubMed]
- Voukeng, I.K.; Beng, V.P.; Kuete, V. Antibacterial activity of six medicinal Cameroonian plants against Gram-positive and Gram-negative multidrug resistant phenotypes. BMC Complement. Altern. Med. 2016, 16, 388. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D.; Sęczyk, Ł.; Złotek, U.; Różyło, R.; Kaszuba, K.; Ryszawy, D.; Czyż, J. Anticancer and antioxidant activity of bread enriched with broccoli sprouts. BioMed Res. Int. 2014, 2014, 608053. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Silván, J.M.; Medina, S.; de Pascual-Teresa, S.; García-Viguera, C.; Moreno, D.A. Metabolism and antiproliferative effects of sulforaphane and broccoli sprouts in human intestinal (Caco-2) and hepatic (HepG2) cells. Phytochem. Rev. 2015, 14, 1035–1044. [Google Scholar] [CrossRef]
- Li, Y.; Buckhaults, P.; Li, S.; Tollefsbol, T. Temporal efficacy of a sulforaphane-based broccoli sprout diet in prevention of breast cancer through modulation of epigenetic mechanisms. Cancer Prev. Res. 2018, 11, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Hinds, L.; Kenny, O.; Hossain, M.; Walsh, D.; Sheehy, E.; Evans, P.; Gaffney, M.; Rai, D. Evaluating the antibacterial properties of polyacetylene and glucosinolate compounds with further identification of their presence within various carrot (Daucus carota) and Broccoli (Brassica oleracea) cultivars using high-performance liquid chromatography with a diode array detector and ultra performance liquid chromatography–tandem mass spectrometry analyses. J. Agric. Food Chem. 2017, 65, 7186–7191. [Google Scholar] [PubMed]
- Moon, J.-K.; Kim, J.-R.; Ahn, Y.-J.; Shibamoto, T. Analysis and anti-Helicobacter activity of sulforaphane and related compounds present in broccoli (Brassica oleracea L.) sprouts. J. Agric. Food Chem. 2010, 58, 6672–6677. [Google Scholar] [CrossRef]
- Corrêa, C.; Martin, J.; Alencar, S.M.D.; Porto, E. Antilisterial activity of broccoli stems (Brassica oleracea) by flow cytometry. Int. Food Res. J. 2014, 21, 395–399. [Google Scholar]



| Germination Time | Total Phenolic Content (mg GAE/g DW) | Total Flavonoid Content (mg CE/g DW) | Vitamin C Content (mg AA/g DW) |
|---|---|---|---|
| Day 3 | |||
| 70% Methanol | 24.21 ± 1.07 cA | 5.10 ± 0.42 dA | 3.39 ± 0.28 bA |
| 70% Ethanol | 20.72 ± 1.86 cB | 4.68 ± 0.15 dB | 2.88 ± 0.34 bAB |
| Hot water | 21.08 ± 1.59 aB | 4.35 ± 0.26 cB | 2.48 ± 0.31 aB |
| Day 5 | |||
| 70% Methanol | 27.44 ± 0.37 aA | 6.08 ± 0.26 cA | 3.62 ± 0.14 abA |
| 70% Ethanol | 29.27 ± 2.12 aA | 5.57 ± 0.22 cB | 3.83 ± 0.12 aA |
| Hot water | 23.31 ± 2.17 aB | 4.88 ± 0.13 bC | 2.63 ± 0.28 aB |
| Day 8 | |||
| 70% Methanol | 27.07 ± 0.78 aB | 7.26 ± 0.26 aA | 3.59 ± 0.14 abA |
| 70% Ethanol | 28.60 ± 0.80 aA | 7.30 ± 0.34 aA | 3.78 ± 0.26 aA |
| Hot water | 21.75 ± 1.27 aC | 4.96 ± 0.12 bB | 2.62 ± 0.23 aB |
| Day 10 | |||
| 70% Methanol | 25.83 ± 0.63 bA | 6.68 ± 0.32 bA | 3.79 ± 0.30 abA |
| 70% Ethanol | 23.54 ± 1.13 bB | 6.83 ± 0.26 bA | 3.29 ± 0.28 abAB |
| Hot water | 17.70 ± 1.48 bC | 5.67 ± 0.11 aB | 2.91 ± 0.25 aB |
| Day 12 | |||
| 70% Methanol | 22.59 ± 0.89 dA | 5.90 ± 0.13 cB | 4.04 ± 0.35 aA |
| 70% Ethanol | 21.53 ± 1.15 cA | 7.05 ± 0.09 abA | 3.31 ± 0.28 abA |
| Hot water | 12.90 ± 0.49 cB | 4.85 ± 0.10 bC | 2.43 ± 0.32 aB |
| Germination Time | DPPH Scavenging Activity (Inhibition %) | Reducing Power Absorbance (700 nm) | ABTS Scavenging Activity (μmol TE/g DW) |
|---|---|---|---|
| Day 3 | |||
| 70% Methanol | 88.06 ± 1.61 aA | 1.90 ± 0.03 aB | 68.60 ± 0.13 aB |
| 70% Ethanol | 91.90 ± 0.67 aA | 1.96 ± 0.01 aA | 69.10 ± 0.13 aA |
| Hot water | 76.24 ± 5.35 aB | 1.48 ± 0.07 aC | 65.91 ± 0.64 aC |
| Day 5 | |||
| 70% Methanol | 83.02 ± 0.58 bB | 1.73 ± 0.04 bB | 68.56 ± 0.17 aA |
| 70% Ethanol | 90.59 ± 0.44 aA | 1.81 ± 0.02 bA | 68.81 ± 0.25 abA |
| Hot water | 56.57 ± 4.57 bC | 1.00 ± 0.05 bC | 65.83 ± 0.55 aB |
| Day 8 | |||
| 70% Methanol | 82.38 ± 0.53 bB | 1.71 ± 0.02 bA | 68.04 ± 0.27 bA |
| 70% Ethanol | 88.82 ± 1.27 aA | 1.70 ± 0.01 cA | 68.61 ± 0.23 bA |
| Hot water | 55.09 ± 0.86 bC | 1.00 ± 0.06 bB | 65.06 ± 0.99 aB |
| Day 10 | |||
| 70% Methanol | 75.43 ± 2.62 cB | 1.60 ± 0.02 cA | 66.65 ± 0.27 cB |
| 70% Ethanol | 83.80 ± 2.79 bA | 1.58 ± 0.02 dB | 68.53 ± 0.20 bA |
| Hot water | 41.11 ± 2.71 cC | 0.49 ± 0.03 cC | 61.94 ± 1.31 bC |
| Day 12 | |||
| 70% Methanol | 69.46 ± 3.13 dB | 1.47 ± 0.02 dB | 65.73 ± 0.42 dB |
| 70% Ethanol | 81.27 ± 2.89 bA | 1.50 ± 0.05 eA | 67.46 ± 0.68 cA |
| Hot water | 40.04 ± 4.87 cC | 0.40 ± 0.02 dC | 59.60 ± 0.97 cC |
| Ascorbic acid * | 96.31 ± 1.08 | 2.59 ± 0.04 | 66.86 ± 0.21 |
| Cell Lines | Cell Growth (%) in Different BSE Concentrations (mg/mL) | IC50 (mg/mL) a | ||||
|---|---|---|---|---|---|---|
| 0.063 | 0.125 | 0.250 | 0.500 | BSE | Cisplatin b | |
| A549 | ||||||
| 24 h | 68.31 ± 4.14 | 61.35 ± 3.09 | 45.09 ± 4.76 | 39.57 ± 3.61 | 0.226 ± 0.022 | 0.009 ± 0.001 |
| 48 h | 61.12 ± 6.98 | 47.56 ± 9.32 | 34.72 ± 3.04 | 26.40 ± 2.19 | 0.117 ± 0.026 | <0.006 |
| HepG2 | ||||||
| 24 h | 84.63 ± 8.86 | 80.76 ± 7.09 | 57.97 ± 5.62 | 40.63 ± 6.00 | 0.355 ± 0.033 | 0.019 ± 0.001 |
| 48 h | 70.21 ± 4.41 | 55.76 ± 5.56 | 39.07 ± 6.60 | 31.58 ± 4.27 | 0.168 ± 0.019 | 0.015 ± 0.003 |
| Caco-2 | ||||||
| 24 h | 75.98 ± 5.79 | 70.36 ± 6.08 | 56.02 ± 3.27 | 41.89 ± 2.61 | 0.338 ± 0.019 | 0.023 ± 0.002 |
| 48 h | 69.51 ± 4.94 | 57.35 ± 5.83 | 45.15 ± 3.82 | 34.96 ± 3.25 | 0.189 ± 0.013 | 0.007 ± 0.003 |
| FL83B | ||||||
| 24 h | 113.89 ± 3.85 | 115.56 ± 3.95 | 103.69 ± 6.54 | 104.12 ± 7.29 | >0.500 | >0.050 |
| 48 h | 103.45 ± 9.10 | 99.96 ± 7.44 | 94.69 ± 8.96 | 96.90 ± 6.20 | >0.500 | 0.027 ± 0.005 |
| Microorganism | Diameter of the Inhibition Zones (mm) a | |||
|---|---|---|---|---|
| BSE | Amp | Amo | D20 | |
| Gram-positive | ||||
| Staphylococcus aureus | 18.36 ± 1.61 | 36.38 ± 0.76 | 33.76 ± 0.56 | ND |
| Bacillus subtilis | 26.44 ± 1.07 | 38.10 ± 0.75 | 37.33 ± 1.39 | ND |
| Gram-negative | ||||
| Escherichia coli | 17.84 ± 0.77 | 39.04 ± 0.87 | 36.00 ± 0.60 | ND |
| Salmonella Typhimurium | 21.03 ± 0.34 | 43.54 ± 0.19 | 40.84 ± 0.47 | ND |
| Microismorgan | Concentration (mg/mL) | NT | Amp | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.10 | 0.2 | 0.39 | 0.78 | 1.56 | 3.13 | 6.25 | 12.5 | 25 | 50 | |||
| Gram-positive | ||||||||||||
| Staphylococcus aureus | + | + | + | + | − | − | − | − | − | − | + | − |
| Bacillus subtilis | + | + | − | − | − | − | − | − | − | − | + | − |
| Gram-negative | ||||||||||||
| Escherichia coli | + | + | + | + | − | − | − | − | − | − | + | − |
| Salmonella Typhimurium | + | + | + | − | − | − | − | − | − | − | + | − |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.N.; Luong, H.Q.; Li, H.-P.; Chiu, C.-H.; Hsieh, P.-C. Broccoli (Brassica oleracea L. var. italica) Sprouts as the Potential Food Source for Bioactive Properties: A Comprehensive Study on In Vitro Disease Models. Foods 2019, 8, 532. https://doi.org/10.3390/foods8110532
Le TN, Luong HQ, Li H-P, Chiu C-H, Hsieh P-C. Broccoli (Brassica oleracea L. var. italica) Sprouts as the Potential Food Source for Bioactive Properties: A Comprehensive Study on In Vitro Disease Models. Foods. 2019; 8(11):532. https://doi.org/10.3390/foods8110532
Chicago/Turabian StyleLe, Thanh Ninh, Hong Quang Luong, Hsin-Ping Li, Chiu-Hsia Chiu, and Pao-Chuan Hsieh. 2019. "Broccoli (Brassica oleracea L. var. italica) Sprouts as the Potential Food Source for Bioactive Properties: A Comprehensive Study on In Vitro Disease Models" Foods 8, no. 11: 532. https://doi.org/10.3390/foods8110532