Bioactive Compounds from Norway Spruce Bark: Comparison Among Sustainable Extraction Techniques for Potential Food Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wood Materials and Chemicals
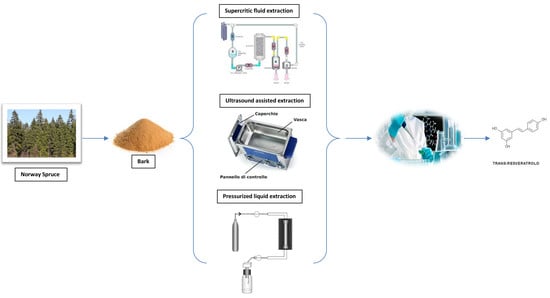
2.2. Supercritical Fluid Extraction
2.3. Pressurized Liquid Extraction
2.4. Ultrasound-Assisted Extraction
2.5. Chemical Characterization
2.5.1. Total Extraction Yield (TEY)
2.5.2. Total Phenolic Content (TPC)
2.5.3. Total Flavonoid Content (TFC)
2.5.4. Antioxidant Activity
2.6. HPLC Analysis of Trans-Resveratrol
2.7. Statistical Analysis
3. Results and Discussion
3.1. Supercritical Fluid Extraction
3.2. Pressurized Liquid and Ultrasound-Assisted Extraction
3.3. Chromatographic Identification of Trans-RSV
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jablonsky, M.M.; Vernarecovà, A.; Haz, L.; Dubinyovà, S.; Andrea, A.; Slàdkovà, I.; Surina, J. Extraction of phenolic and lipophilic compounds from spruce (Picea abies) bark using accelerated solvent extraction by ethanol. Wood Res. 2015, 6, 583–590. [Google Scholar]
- Ekman, A.; Campos, M.S.; Lindhal, M.; Co, P.; Borjesson, E.N.; Karlsson, E.N.; Turner, C. Bioresource utilisation by sustainable technologies in new value-added biorefinery concepts -two case studies from food and forest industry. J. Clean. Prod. 2013, 57, 46–58. [Google Scholar] [CrossRef]
- Co, M.; Fagerlund, A.; Engman, L.; Sunnerheim, K.; Sjoberg, P.J.; Turner, C. Extraction of antioxidants from spruce (Picea abies) bark using eco-friendly solvents. Phytochem. Anal. 2012, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Pan, H.; Lundgren, L.N. Phenolic extractives from root bark of Picea abies. Phytochemical 1995, 39, 1423–1428. [Google Scholar] [CrossRef]
- Gabaston, J.; Richard, T.; Biais, B.; Waffo-Teguo, P.; Pedrot, E.; Jourdes, M.; Corio-Costet, M.F.; Mèrillon, M.J. Stilbenes from common spruce (Picea abies) bark as natural antifungal agent against downy mildew (Plasmopara viticola). Ind. Crops Prod. 2017, 103, 267–273. [Google Scholar] [CrossRef]
- Hasan, M.; Bae, H. An overview of stress-induced resveratrol synthesis in grapes: Perspectives for resveratrol-enriched grape products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef]
- Smoliga, J.M.; Baur, J.A.; Hausenblas, H. Resveratrol and health: A comprehensive review of human clinical trials. Mol. Nutr. Food Res. 2011, 55, 1129–1141. [Google Scholar] [CrossRef]
- Kursvietiene, L.; Staneviciene, I.; Morgidiene, A.; Bernatoniene, J. Multiplicity of effects and health benefits of resveratrol. Medicina 2016, 52, 148–155. [Google Scholar] [CrossRef]
- Szkudelska, K.; Szkudelski, T. Resveratrol, obesity and diabetes. Eur. J. Pharm. 2010, 635, 1–8. [Google Scholar] [CrossRef]
- Fogacci, F.; Tocci, G.; Presta, V.; Fratter, A.; Borghi, C.; Cicero, A.F. Effect of resveratrol on blood pressure: A systematic review and meta-analysis of randomized, controlled, clinical trials. Crit. Rev. Food Sci. Nutr. 2018, 59, 1–14. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Ratz-Łyko, A.; Arct, J. Resveratrol as an active ingredient for cosmetic and dermatological applications: A review. J. Cosmet. Laser Ther. 2019, 21, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Talmaciu, A.I.; Ravber, M.; Volf, I.; Knez, Z.; Popa, V. Isolation of bioactive compounds from spruce bark waste using sub- and supercritical fluids. J. Supercrit. Fluids 2016, 117, 243–251. [Google Scholar] [CrossRef]
- Ghitescu, R.E.; Volf, I.; Carausu, C.; Buhlmann, A.M.; Glica, I.A.; Popa, V. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef]
- Hatti-Kaul, R.; Tornvall, U.; Gustafsson, L.; Borjesson, P. Industrial biotechnology for the production of bio-based chemicals—A cradle-to-grave perspective. Trends Biotechnol. 2007, 25, 119–124. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibanez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Garcìa-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic compound extraction systems for fruit and vegetable samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef]
- Pereira, C.G.; Meireles, M.A.A. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Nautyal, O.H. Food processing by supercritical carbon dioxide-review. EC Chem. 2016, 21, 111–135. [Google Scholar]
- Spinelli, S.; Conte, A.; Lecce, L.; Padalino, L.; Del Nobile, M.A. Supercritical carbon dioxide extraction of brewer’s spent grain. J. Supercrit. Fluids 2016, 107, 69–74. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.T.; Veggi, P.C.; Meireles, M.A.A. Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. J. Food Eng. 2012, 108, 444–452. [Google Scholar] [CrossRef] [Green Version]
- Marinelli, V.; Padalino, L.; Nardiello, D.; Del Nobile, M.A.; Conte, A. New approach to enrich pasta with polyphenols from grape marc. J. Chem. 2015, 8, 1–8. [Google Scholar] [CrossRef]
- Lucera, A.; Costa, C.; Marinelli, V.; Saccotelli, M.A.; Del Nobile, M.A.; Conte, A. Fruit and Vegetable By-Products to Fortify Spreadable Cheese. Antioxidants 2018, 7, 61. [Google Scholar] [CrossRef]
- Sun, Y.; Qi, Y.; Mu, Z.; Wang, K. Quantitative determination of resveratrol in Polygonum cuspidatum and its anti-proliferative effect on melanoma A375 cells. Biomed. Res. 2015, 26, 750–754. [Google Scholar]
- Veggi, P.C.; Prado, J.M.; Bataglioni, G.A.; Eberlin, M.N.; Meireles, M.A.A. Obtaining phenolic compounds from jatoba (Hymenaea courbaril L.) bark by supercritical fluid extraction. J. Supercrit. Fluids 2014, 89, 68–77. [Google Scholar] [CrossRef]
- Salleh, L.M.; Rahman, R.A.; Selamat, J.; Hamid, A.; Sarker, M.Z.I. Optimization of extraction condition for supercritical carbon dioxide (SC-CO2) extraction of Strobhilantes Crispus (Pecah Kaca) leaves by response surface methodology. J. Food Process Technol. 2013, 4, 197–201. [Google Scholar]
- Conde, E.; Hemming, J.; Smeds, A.; Reinoso, B.D.; Moure, A.; Willfor, S.; Domìnguez, H.; Parajò, J.C. Extraction of low-molar-mass phenolics and lipophilic compounds from Pinus pinaster wood with compressed CO2. J. Supercrit. Fluids 2013, 81, 193–199. [Google Scholar] [CrossRef]
- Fabrowska, J.; Ibanez, E.; Leska, B.; Herrero, M. Supercritical fluid extraction as a tool to valorize underexploited freshwater green algae. Algal Res. 2016, 19, 237–245. [Google Scholar] [CrossRef]
- Khaw, K.Y.; Parat, M.O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef] [PubMed]
- Zaidulism, J.A.; Rahman, M.M.; Sharis, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Noriliani, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Pressurized liquid extraction of isoflavones from soybeans. Anal. Chim. Acta 2004, 522, 169–177. [Google Scholar] [CrossRef]
- Howard, L.; Pandjaitan, N. Pressurized liquid extraction of flavonoids from spinach. J. Food Sci. 2008, 73, 151–157. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, J.; Lu, J.; Chen, J.; Li, Y.; Shan, L.; Lin, Y.; Fan, W.; Gu, G. Effects of extraction solvent mixtures on antioxidant activity evaluation and their extraction capacity and selectivity for free phenolic compounds in barley (Hordeum vulgare L.). J. Agric. Food Chem. 2006, 54, 7277–7286. [Google Scholar] [CrossRef]
- Hemwimol, S.; Pavasant, P.; Shotipruk, A. Ultrasound-assisted extraction of anthraquinones from roots of Morinda citrifolia. Ultrason. Sonochem. 2006, 13, 543–548. [Google Scholar] [CrossRef]
- Thaiponga, K.; Boonprakoba, U.; Crosbyb, K.; Cisneros-Zevallosc, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Youn, J.S.; Kim, Y.J.; Na, H.J.; Jung, H.R.; Song, C.K.; Kang, S.Y.; Kim, J.Y. Antioxidant activity and contents of leaf extracts obtained from Dendropanax morbifera LEV are dependent on the collecting season and extraction conditions. Food Sci. Biotechnol. 2019, 28, 201–207. [Google Scholar] [CrossRef]
- Teixeira, T.S.; Vale, R.C.; Almeida, R.R.; Ferreira, T.P.S.; Guimarães, L.G.L. Antioxidant Potential and its Correlation with the Contents of Phenolic Compounds and Flavonoids of Methanolic Extracts from Different Medicinal Plants. Rev. Virtual Quim. 2017, 9, 1546–1559. [Google Scholar] [CrossRef]
- Chaudhari, G.M.; Mahajan, R.T. Comparative Antioxidant Activity of Twenty Traditional Indian Medicinal Plants and its Correlation with Total Flavonoid and Phenolic Content. Int. J. Pharm. Sci. Rev. Res. 2015, 30, 105. [Google Scholar]
- Kareem, H.S.; Ariffin, A.; Nordin, N.; Heidelberg, T.; Abdul-Aziz, A.; Kong, K.W.; Yehye, W.A. Correlation of antioxidant activities with theoretical studies for new hydrazone compounds bearing a 3,4,5-trimethoxy benzyl moiety. Eur. J. Med. Chem. 2015, 103, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Du, F.Y.; Xiao, X.H.; Li, J.K. Application of ionic liquids in the microwave-assisted extraction of trans-resveratrol from Rhizma Polygoni Cuspidati. J. Chromatogr. A 2007, 1140, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Zupancic, S.; Lavric, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Garcìa-Pèrez, M.E.; Royer, M.; Herbette, G.; Desjardins, Y.; Pouliot, R.; Stevanovic, T. Picea mariana bark: A new source of trans-resveratrol and other bioactive polyphenols. Food Chem. 2012, 135, 1173–1182. [Google Scholar] [CrossRef] [PubMed]

| TEY | TPC | TFC | ABTS | FRAP | |
|---|---|---|---|---|---|
| mg/g dw | mg GAEs/g dw | mg QEs/g dw | mg TEs/g dw | µmol FeSO4·7H2O/g dw | |
| SFE_10 | 28.6 ± 0.36 a | 0.77 ± 0.02 a | 0.47 ± 0.02 a | 2.48 ± 0.13 a | 8.31 ± 0.24 a |
| SFE_20 | 30.7 ± 0.96 a | 1.24 ± 0.07 b | 1.05 ± 0.15 b | 3.08 ± 0.16 b | 10.01 ± 0.81 b |
| SFE_40 | 31.2 ± 0.21 a | 2.50 ± 0.03 c | 1.75 ± 0.10 c | 5.29 ± 0.04 c | 25.49 ± 0.66 c |
| TEY | TPC | TFC | ABTS | FRAP | |
|---|---|---|---|---|---|
| mg/g dw | mg GAEs/g dw | mg QEs/g dw | mg TEs/g dw | µmol FeSO4·7H2O/g dw | |
| PLE_H2O | 130.7 ± 8.62 a | 33.45 ± 1.44 a | 19.03 ± 0.98 a | 69.87 ± 1.46 c | 389.10 ± 16.87 b |
| PLE_EtOH | 127.9 ± 2.52 a | 46.32 ± 2.17 b | 21.14 ± 1.42 a | 257.11 ± 13.31 a | 506.10 ± 31.37 a |
| UAE_EtOH | 123.3 ± 5.77 a | 54.97 ± 2.00 c | 14.44 ± 1.31 b | 128.47 ± 8.61 b | 580.25 ± 25.18 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spinelli, S.; Costa, C.; Conte, A.; La Porta, N.; Padalino, L.; Del Nobile, M.A. Bioactive Compounds from Norway Spruce Bark: Comparison Among Sustainable Extraction Techniques for Potential Food Applications. Foods 2019, 8, 524. https://doi.org/10.3390/foods8110524
Spinelli S, Costa C, Conte A, La Porta N, Padalino L, Del Nobile MA. Bioactive Compounds from Norway Spruce Bark: Comparison Among Sustainable Extraction Techniques for Potential Food Applications. Foods. 2019; 8(11):524. https://doi.org/10.3390/foods8110524
Chicago/Turabian StyleSpinelli, Sara, Cristina Costa, Amalia Conte, Nicola La Porta, Lucia Padalino, and Matteo Alessandro Del Nobile. 2019. "Bioactive Compounds from Norway Spruce Bark: Comparison Among Sustainable Extraction Techniques for Potential Food Applications" Foods 8, no. 11: 524. https://doi.org/10.3390/foods8110524