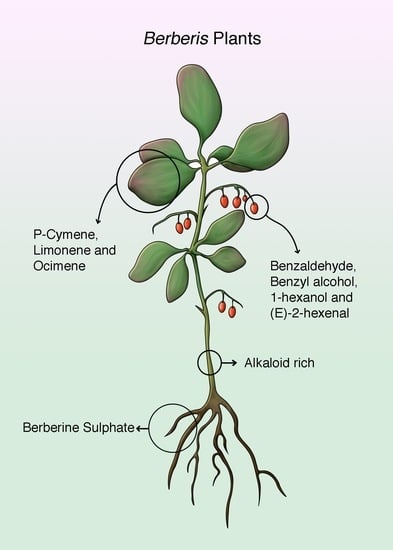
Berberis Plants—Drifting from Farm to Food Applications, Phytotherapy, and Phytopharmacology
Abstract
:1. Introduction
2. Cultivation of Berberis Plants
3. Berberis Plants Essential Oils and Phytochemical Composition
4. Food Preservative Applications of Berberis Plants
5. Antioxidant Activities of Berberis Plants (In Vitro and In Vivo)
6. Anticancer Activities of Berberis Plants (In Vitro and In Vivo)
7. Clinical Studies of Berberis Plants in Human
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Minaiyan, M.; Ghannadi, A.; Mahzouni, P.; Jaffari-Shirazi, E. Comparative study of berberis vulgaris fruit extract and berberine chloride effects on acetic acid-induced colitis in rats. Iran. J. Pharm. Res. 2011, 10, 97–104. [Google Scholar] [PubMed]
- Mokhber-Dezfuli, N.; Saeidnia, S.; Gohari, A.; Kurepaz-Mahmoodabadi, M. Phytochemistry and Pharmacology of Berberis Species. Pharmacogn. Rev. 2014, 8, 8. [Google Scholar] [PubMed]
- Rahimi-Madiseh, M.; Lorigoini, Z.; Zamani-Gharaghoshi, H.; Rafieian-Kopaei, M. Berberis vulgaris: Specifications and traditional uses. Iran. J. Basic Med. Sci. 2017, 20, 569–587. [Google Scholar] [PubMed]
- Farhadi Chitgar, M.; Aalami, M.; Maghsoudlou, Y.; Milani, E. Comparative Study on the Effect of Heat Treatment and Sonication on the Quality of Barberry (Berberis Vulgaris) Juice. J. Food Process. Preserv. 2017, 41, e12956. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Kianmehr, M.H.; Hassan-Beygi, S.R. Specific heat and thermal conductivity of berberis fruit (Berberis vulgaris). Am. J. Agric. Biol. Sci. 2008, 3, 330–336. [Google Scholar] [CrossRef]
- Birdsall, T.C.; Kelly, G.S. Berberine: Therapeutic potential of an alkaloid found in several medicinal plants. Altern. Med. Rev. 1997, 2, 94–103. [Google Scholar]
- Kang, J.; Kang, Y.; Ji, X.; Guo, Q.; Jacques, G.; Pietras, M.; Łuczaj, N.; Li, D.; Łuczaj, Ł. Wild food plants and fungi used in the mycophilous Tibetan community of Zhagana (Tewo County, Gansu, China). J. Ethnobiol. Ethnomed. 2016, 12, 21. [Google Scholar] [CrossRef]
- Tavakoli, A.; Sahari, M.A.; Barzegar, M. Antioxidant activity of Berberis integerrima seed oil as a natural antioxidant on the oxidative stability of soybean oil. Int. J. Food Prop. 2018, 20, S2914–S2925. [Google Scholar] [CrossRef]
- Aliakbarlu, J.; Mohammadi, S.; Khalili, S. A Study on Antioxidant Potency and Antibacterial Activity of Water Extracts of Some Spices Widely Consumed in Iranian Diet. J. Food Biochem. 2013, 38, 159–166. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, M.; Misra, A.; Pandey, G.; Rawat, A. A review on biological and chemical diversity in Berberis (Berberidaceae). EXCLI J. 2015, 14, 247–267. [Google Scholar]
- Kafi, M.; Balandary, A.; Rashed-Mohasel, M.H.; Koochaki, A.; Molafilabi, A. Berberis: Production and Processing; Zaban va adab Press: City, Iran, 2002; ISBN 9789290814993. [Google Scholar]
- Saied, S.; Begum, S. Phytochemical studies of Berberis vulgaris. Chem. Nat. Compd. 2004, 40, 137–140. [Google Scholar] [CrossRef]
- Yazdani, A.; Poorbaghi, S.L.; Habibi, H.; Nazifi, S.; Rahmani Far, F.; Sepehrimanesh, M. Dietary Berberis vulgaris extract enhances intestinal mucosa morphology in the broiler chicken (Gallus gallus). Comp. Clin. Path. 2013, 22, 611–615. [Google Scholar] [CrossRef]
- Bashir, S.; Gilani, A.H.; Siddiqui, A.A.; Pervez, S.; Khan, S.R.; Sarfaraz, N.J.; Shah, A.J. Berberis vulgaris root bark extract prevents hyperoxaluria induced urolithiasis in rats. Phyther. Res. 2010, 24, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Hermenean, A.; Popescu, C.; Ardelean, A.; Stan, M.; Hadaruga, N.; Mihali, C.V.; Costache, M.; Dinischiotu, A. Hepatoprotective effects of Berberis vulgaris L. extract/β cyclodextrin on carbon tetrachloride-induced acute toxicity in mice. Int. J. Mol. Sci. 2012, 13, 9014–9034. [Google Scholar] [CrossRef] [PubMed]
- Amjad, M.S.; Arshad, M.; Qureshi, R. Ethnobotanical inventory and folk uses of indigenous plants from Pir Nasoora National Park, Azad Jammu and Kashmir. Asian Pac. J. Trop. Biomed. 2015, 5, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Altundag, E.; Ozturk, M. Ethnomedicinal studies on the plant resources of east Anatolia, Turkey. Procedia Soc. Behav. Sci. 2011, 19, 756–777. [Google Scholar] [CrossRef] [Green Version]
- Javadzadeh, S.; Fallah, S. Therapeutic application of different parts of Berberis vulgaris. Int. J. Agric. Crop Sci. 2012, 4, 404–408. [Google Scholar]
- Phillips, R.; Foy, N. Herbs; Pan Books Ltd.: London, UK, 2002; ISBN 0-330-30725-8. [Google Scholar]
- Kuo, C.-L.; Chi, C.-W.; Liu, T.-Y. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004, 203, 127–137. [Google Scholar] [CrossRef]
- Sun, D.; Courtney, H.S.; Beachey, E.H. Berberine sulfate blocks adherence of Streptococcus pyogenes to epithelial cells, fibronectin, and hexadecane. Antimicrob. Agents Chemother. 1988, 32, 1370–1374. [Google Scholar] [CrossRef] [Green Version]
- Hajzadeh, M.A.R.; Rajaei, Z.; Shafiee, S.; Alavinejhad, A.; Samarghandian, S.; Ahmadi, M. Effect of barberry fruit (Berberis Vulgaris) on serum glucose and lipids in streptozotocin-diabetic rats. Pharmacologyonline 2011, 1, 809–817. [Google Scholar]
- Meliani, N.; Dib, M.E.A.; Allali, H.; Tabti, B. Hypoglycaemic effect of Berberis vulgaris L. in normal and streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Biomed. 2011, 1, 468–471. [Google Scholar] [CrossRef]
- Zhou, X.; Chan, S.W.; Tseng, H.L.; Deng, Y.; Hoi, P.M.; Choi, P.S.; Or, P.M.Y.; Yang, J.M.; Lam, F.F.Y.; Lee, S.M.Y.; et al. Danshensu is the major marker for the antioxidant and vasorelaxation effects of Danshen (Salvia miltiorrhiza) water-extracts produced by different heat water-extractions. Phytomedicine 2012, 19, 1263–1269. [Google Scholar] [CrossRef]
- Zhu, X.; Bian, H.; Gao, X. The Potential Mechanisms of Berberine in the Treatment of Nonalcoholic Fatty Liver Disease. Molecules 2016, 21, 1336. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yin, J.; Gao, H.; Xu, L.; Wang, Y.; Xu, L.; Li, M. Berberine Improves Insulin Sensitivity by Inhibiting Fat Store and Adjusting Adipokines Profile in Human Preadipocytes and Metabolic Syndrome Patients. Evid.-Based Complement. Altern. Med. 2012, 2012, 363845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, J. Mitochondrial inhibitor as a new class of insulin sensitizer. Acta Pharm. Sin. B 2012, 2, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Imenshahidi, M.; Hosseinzadeh, H. Berberis Vulgaris and Berberine: An Update Review. Phyther. Res. 2016, 30, 1745–1764. [Google Scholar] [CrossRef]
- West, S.; King, V.; Carey, T.S.; Lohr, K.N.; McKoy, N.; Sutton, S.F.; Lux, L. Systems to rate the strength of scientific evidence. Evid. Rep. Technol. Assess. 2002, 47, 1–11. [Google Scholar]
- Rounsaville, T.J.; Ranney, T.G. Ploidy levels and genome sizes of berberis l. and mahonia nutt. species, hybrids, and cultivars. HortScience 2010, 45, 1029–1033. [Google Scholar] [CrossRef]
- Mozaffarian, V. A Dictionary of Iranian Plant Names; Farhang Mo’aser: Tehran, Iran, 2008; ISBN 9645545196. [Google Scholar]
- Kafi, M.; Balandri, A. Effects of gibberellic acid and ethephon on fruit characteristics and ease of harvest seed less barberry. Iran. Res. Organ. Sci. Technol. Cent. Khorasan 1995, volume, page. [Google Scholar]
- Peterson, P.; Leonard, K.; Miller, J.; Laudon, R.; Sutton, T. Prevalence and distribution of common barberry, the alternate host of Puccinia graminis, in Minnesota. Plant Dis. 2005, 89, 159–163. [Google Scholar] [CrossRef]
- Łuczaj, L. Archival data on wild food plants used in Poland in 1948. J. Ethnobiol. Ethnomed. 2008, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, Ł. Wild food plants used in Poland from the mid-19th century to the present. [Dziko rosnące rośliny jadalne użytkowane w Polsce od połowy XIX w. do czasów współczesnych]. Etnobiologia Pol. 2011, 1, 57–125. [Google Scholar]
- Bussmann, R.; Zambrana, P.; Narel, Y.; Sikharulidze, S.; Kikvidze, Z.; Kikodze, D.; Tchelidze, D.; Batsatsashvili, K.; Robbie, E. Ethnobotany of Samtskhe-Javakheti, Sakartvelo (Republic of Georgia), Caucasus. Indian J. Tradit. Knowl. 2017, 12, 7–24. [Google Scholar]
- Kern, F.D. Observations of the Dissemination of the Barberry. Ecology 1921, 2, 211–214. [Google Scholar] [CrossRef]
- Dirr, M. Manual of Woody Landscape Plants: Their Identification, Ornamental Characteristics, Culture, Propagation, and Uses, 5th ed.; Stipes Publishing: Champaign, IL, USA, 1998; ISBN 9781588748706. [Google Scholar]
- Mack, R.N.; Erneberg, M. The United States naturalized flora: Largely the product of deliberate introductions. Ann. Missouri Bot. Gard. 2002, 89, 176–189. [Google Scholar] [CrossRef]
- Fulling, E.H. Plant life and the law of man IV barberry, currant and gooseberry, and cedar control. Bot. Rev. 1943, 9, 483–592. [Google Scholar] [CrossRef]
- Javadzadeh, S. Effect of different methods of harvesting, drying and time on losses seedless barberry (Berberis vulgaris L). Int. J. Agron. Plant 2013, 4, 254–260. [Google Scholar]
- Sharma, R. Medicinal plants of India: An Encyclopaedia. Indian Counc. Med. Res. New Delhi 2003, 1, 33. [Google Scholar]
- Li, X.; Zhang, L.; Li, W.; Yin, X.; Yuan, S. New taxa of Berberis (Berberidaceae) with greenish flowers from a biodiversity hotspot in Sichuan Province, China. Plant Divers. 2017, 39, 94–103. [Google Scholar] [CrossRef]
- Ward, J.S.; Worthley, T.E.; Williams, S.C. Controlling Japanese barberry (Berberis thunbergii DC) in southern New England, USA. For. Ecol. Manag. 2009, 257, 561–566. [Google Scholar] [CrossRef]
- Adhikari, B.; Pendry, C.A.; Pennington, R.T.; Milne, R.I. A revision of berberis S.S. (Berberidaceae) in Nepal. Edinburgh J. Bot. 2012, 69, 447–522. [Google Scholar] [CrossRef]
- Baytop, T. Turkish Plant Names Dictionary. Atatürk Culture, Language and History High Foundation; Turkish Language Foundation: Ankara, Turkey, 1994. [Google Scholar]
- Khan, T.; Khan, I.A.; Rehman, A. A review on Berberis species reported from Gilgit- Baltistan and Central Karakoram National Park, Pakistan. J. Med. Plants Stud. 2014, 2, 16–20. [Google Scholar]
- Ali, M.; Malik, A.R.; Sharma, K.R. Vegetative propagation of Berberis aristata DC. An endangered Himalayan shrub. J. Med. Plants 2008, 2, 374–377. [Google Scholar]
- Ali, M.N.; Khan, A.A. Pharmacognostic studies on Berberis lycium Royle, and its importance as a source of raw material for the manufacture of berberine in Pakistan [angiosperm trees]. Pakistan J. For. 1978, 28, 25–27. [Google Scholar]
- Kaur, C.; Miani, S. Fruits and vegetables healthy foods for new millennium. Indian Hort 2001, 45, 29–32. [Google Scholar]
- Tewary, D.K.; Bhardwaj, A.; Shanker, A. Pesticidal activities in five medicinal plants collected from mid hills of western Himalayas. Ind. Crops Prod. 2005, 22, 241–247. [Google Scholar] [CrossRef]
- Fallahi, J.; Moghaddam, R.P.; Nasiri-Mahallati, M. Effect of harvest date on quantitative and qualitative indices of seedless barberry. Iran. J. F. Crop. Res. 2010, 8, 225–234. [Google Scholar]
- Moghaddam, P.R.; Fallahi, J.; Shajari, M.A.; Mahallati, M.N. Effects of harvest date, harvest time, and post-harvest management on quantitative and qualitative traits in seedless barberry (Berberis vulgaris L.). Ind. Crops Prod. 2013, 42, 30–36. [Google Scholar] [CrossRef]
- Arena, M.E.; Curvetto, N. Berberis buxifolia fruiting: Kinetic growth behavior and evolution of chemical properties during the fruiting period and different growing seasons. Sci. Hortic. (Amsterdam) 2008, 118, 120–127. [Google Scholar] [CrossRef]
- Chandra, P.; Todaria, N.P. Maturation and ripening of three Berberis species from different altitudes. Sci. Hortic. (Amsterdam) 1983, 19, 91–95. [Google Scholar] [CrossRef]
- Mahmoodi, H.R.; Zamani, G.H.; Balandary, A. The study of qualitative characteristics of seedless barberry (Berberis vulgaris L.) as influenced by different fruit harvesting dates and two different climates. In Proceedings of the 6th Congress of Iranian Horticultural Sciences, Isfahan, Iran, 2009; pp. 1486–1489. [Google Scholar]
- Minore, D.; Rudolf, P.O.; Berberis, L. The Woody Plant Seed Manual, Agriculture Handbook; Bonner, F.T., Karrfalt, R.P., Eds.; U.S. Department of Agriculture Forest Service: Washington, DC, USA, 2008; Volume 727, pp. 298–302. [Google Scholar]
- Obesco, J.R. Fruit removal and potential seed dispersal in a southern Spanish population of Berberis vulgaris subsp. australis (Berberidaceae). Acta Oecologica/Oecologia Pantarum 1989, 10, 321–328. [Google Scholar]
- Royer, F.; Dickinson, R. Weeds of the Northern U.S. and Canada: A Guide for Identification; University of Alberta: Edmonton, AB, Canada, 1999; ISBN 1551052210. [Google Scholar]
- Chapman, W.K.; Bessette, A.E. Trees and Shrubs of the Adirondacks; North Country Books, Inc.: Utica, NY, USA, 1990. [Google Scholar]
- Eriksson, O.; Ehrlén, J. Phenological variation in fruit characteristics in vertebrate-dispersed plants. Oecologia 1991, 86, 463–470. [Google Scholar] [CrossRef]
- Imanshahidi, M.; Hosseinzadeh, H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phyther. Res. 2008, 22, 999–1012. [Google Scholar] [CrossRef]
- Alemardan, A.; Asadi, W.; Rezaei, M.; Tabrizi, L.; Mohammadi, S. Cultivation of Iranian seedless barberry (Berberis integerrima ‘Bidaneh’): A medicinal shrub. Ind. Crops Prod. 2013, 50, 276–287. [Google Scholar] [CrossRef]
- Potdar, D.; Hirwani, R.R.; Dhulap, S. Phyto-chemical and pharmacological applications of Berberis aristata. Fitoterapia 2012, 83, 817–830. [Google Scholar] [CrossRef]
- Dolezal, M.; Velisek, J.; Famfulikova, P. Chemical composition of less-known wild fruits. In Proceedings of the EUROFOODCHEM XI Meeting, Norwich, UK, 26–28 September 2001; Volume 269, pp. 241–244. [Google Scholar]
- Hamedi, A.; Moheimani, S.M.; Sakhteman, A.; Etemadfard, H.; Moein, M. An Overview on Indications and Chemical Composition of Aromatic Waters (Hydrosols) as Functional Beverages in Persian Nutrition Culture and Folk Medicine for Hyperlipidemia and Cardiovascular Conditions. J. Evid.-Based Complement. Altern. Med. 2017, 22, 544–561. [Google Scholar] [CrossRef]
- Sonmezdag, A.S.; Kelebek, H.; Selli, S. Volatile and key odourant compounds of Turkish Berberis crataegina fruit using GC-MS-Olfactometry. Nat. Prod. Res. 2018, 32, 777–781. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Mohammadhosseini, M.; Azizi, Z. Impact of amine- and phenyl-functionalized magnetic nanoparticles impacts on microwave-assisted extraction of essential oils from root of Berberis integerrima Bunge. J. Appl. Res. Med. Aromat. Plants 2018, 10, 1–8. [Google Scholar] [CrossRef]
- Bonesi, M.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Saab, A.; Menichini, F.; Tundis, R. Berberis aetnensis and B. libanotica: A comparative study on the chemical composition, inhibitory effect on key enzymes linked to Alzheimer’s disease and antioxidant activity. J. Pharm. Pharmacol. 2013, 65, 1726–1735. [Google Scholar] [CrossRef]
- Jay, J. Modern Food Microbiology; Aspen Publishers Inc.: Gaithersburg, MD, USA, 1998; ISBN 978-0-387-23180-8. [Google Scholar]
- Pszczola, D.E. Emerging ingredients: Believe it or not! Food Technol. 1999, 53, 98–100. [Google Scholar]
- Kaya, M.; Ravikumar, P.; Ilk, S.; Mujtaba, M.; Akyuz, L.; Labidi, J.; Salaberria, A.M.; Cakmak, Y.S.; Erkul, S.K. Production and characterization of chitosan based edible films from Berberis crataegina’s fruit extract and seed oil. Innov. Food Sci. Emerg. Technol. 2018, 45, 287–297. [Google Scholar] [CrossRef]
- Thusa, R.; Mulmi, S. Analysis of Phytoconstituents and Biological Activities of Different Parts of Mahonia nepalensis and Berberis aristata. Nepal J. Biotechnol. 2017, 5, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Pokhrel, N.R.; Adhikari, R.P.; Baral, M.P. In Vitro screening and evaluation of antimicrobial activities of some medicinal plants of Nepal. Nepal J. Sci. Technol. 2003, 5, 1. [Google Scholar]
- Ebrahimi, A.; Chavoushpour, M.; Mahzoonieh, M.R.; Lotfalian, S. Antibacterial activity and ciprofloxacin-potentiation property of Berberis vulgaris asperma stem extracts on pathogenic bacteria. J. HerbMed Pharmacol. 2016, 5, 112–115. [Google Scholar]
- Singh, S.K.; Vishnoi, R.; Dhingra, G.K.; Kishor, K. Antibacterial activity of leaf extracts of some selected traditional medicinal plants of Uttarakhand, North East India. J. Appl. Nat. Sci. 2012, 4, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Saravanakumar, T.; Manonmani, E.; Venkatasubramanian, P.; Vasanthi, N.S. Antimicrobial potential of Daruharidra (Berberis aristata DC) against the pathogens causing eye infection. Int. J. Green Pharm. 2014, 8, 153. [Google Scholar] [CrossRef]
- Alamzeb, M. Bioassay guided isolation and characterization of anti-microbial and anti-trypanosomal agents from Berberis glaucocarpa Stapf. African J. Pharm. Pharmacol. 2013, 7, 2564–2570. [Google Scholar] [CrossRef]
- Parvu, M.; Parvu, A.E.; Craciun, C.; Barbu-Tudoran, L.; Vlase, L.; Tamas, M.; Rosca-Casian, O.; PersecA, O.; Molnar, A.M. Changes in Botrytis cinerea conidia caused by Berberis vulgaris extract. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 15–20. [Google Scholar]
- Shah, Z.; Ilyas, M.; Khan, M.; Ahmad, A.; Khan, M.; Khan, N. Antimicrobial activities of selected medicinal plants collected from Northern districts of Khyber Pakhtunkhwa, Pakistan. J. Pharm. Res. 2012, 5, 1729–1733. [Google Scholar]
- Ghareeb, D.A.; El-Wahab, A.E.A. Biological assessment of Berberis vulgaris and its active constituent, berberine: Antibacterial, antifungal and anti-hepatitis C virus (HCV) effect. J. Med. Plants Res. 2013, 7, 1529–1536. [Google Scholar]
- Shahid, T.; Memon, M.; Malik, R.A.; Ikram, N.; Malik, W.; Ali, A. A study of Antimicrobial Activity of Berberis vulgaris (Zirishk) Aqueous Plant Extract using Pathogenic Isolates from Patients of Islamabad and Rawalpindi. Imp. J. Interdiscip. Res. 2017, 3, 1365–1371. [Google Scholar]
- Krisch, J.; Galgóczy, L.; Tölgyesì, M.; Papp, T.; Vágvölgyi, C. Effect of fruit juices and pomace extracts on the growth of Gram-positive and Gram-negative bacteria. Acta Biol. Szeged. 2008, 52, 267–270. [Google Scholar]
- Rasool, S.; Khan, F.Z.; Hassan, S.U.; Ahmed, M.; Ahmed, M.; Tareen, R.B. Anticonvulsant, antimicrobial and cytotoxic activities of berberis calliobotrys aitch ex koehne (Berberidaceae). Trop. J. Pharm. Res. 2015, 14, 2031–2039. [Google Scholar] [CrossRef]
- Irshad, A.H.; Pervaiz, A.H.; Abrar, Y.B.; Fahelboum, I.; Awen, B.Z.S. Antibacterial activity of Berberis lycium root extract. Trakia J. Sci. 2013, 11, 88–90. [Google Scholar]
- Haouat, A.C.; Haggoud, A.; David, S.; Ibnsouda, S.; Iraqui, M. In vitro evaluation of the antimycobacterial activity and fractionation of Berberis hispanica root bark. J. Pure Appl. Microbiol. 2014, 8, 917–925. [Google Scholar]
- Mattana, C.M.; Satorres, S.E.; Juan, V.; Cifuente, D.; Tonn, C.; Laciar, A.L. Antibacterial activity study of single and combined extracts of Berberis ruscifolia, Baccharis sagittalis, Euphorbia dentata and Euphorbia schikendanzii, native plants from Argentina. BLACPMA 2012, 11, 428–434. [Google Scholar]
- Joshi, P.V.; Shirkhedkar, A.A.; Prakash, K.; Maheshwari, V.L. Antidiarrheal activity, chemical and toxicity profile of Berberis aristata. Pharm. Biol. 2011, 49, 94–100. [Google Scholar] [CrossRef]
- Singh, M.; Srivastava, S.; Rawat, A.K.S. Antimicrobial activities of Indian Berberis species. Fitoterapia 2007, 78, 574–576. [Google Scholar] [CrossRef]
- Hussain, M.A.; Khan, M.Q.; Habib, T.; Hussain, N. Antimicronbial activity of the crude root extract of berberis lycium royle. Adv. Environ. Biol. 2011, 5, 585–588. [Google Scholar]
- Azimi, G.; Hakakian, A.; Ghanadian, M.; Joumaa, A.; Alamian, S. Bioassay-directed isolation of quaternary benzylisoquinolines from Berberis integerrima with bactericidal activity against Brucella abortus. Res. Pharm. Sci. 2018, 13, 149–158. [Google Scholar]
- Bukhari, I.; Hassan, M.; Abbasi, F.; Mujtaba, G.; Mahmood, N.; Fatima, A.; Afzal, M.; Rehman, M.; Perveen, P.; Khan, T. A study on comparative pharmacological efficacy of Berberis lycium and penicillin G. African J. Microbiol. Res. 2011, 5, 725–727. [Google Scholar]
- Sati, S.C.; Takuli, P.; Kumar, P.; Khulbe, K. Antibacterial activity of three medicinal plants of Kumaun Himalaya against some pathogenic bacteria. Int. J. Pharma Sci. Res. 2015, 6, 1361–1368. [Google Scholar]
- Malik, Z.; Jain, K.; Ravindran, K.; Sathiyaraj, G. In vitro antimicrobial activity and preliminary phytochemical analysis of Berberis aristata. Int. J. Ethnobiol. Ethnomed. 2017, 4, 1–6. [Google Scholar]
- Manosalva, L.; Mutis, A.; Urzúa, A.; Fajardo, V.; Quiroz, A. Antibacterial activity of alkaloid fractions from berberis microphylla G. Forst and study of synergism with ampicillin and cephalothin. Molecules 2016, 21, 76. [Google Scholar] [CrossRef]
- Mehmood, A.; Murtaza, G.; Bhatti, T.M.; Kausar, R.; Ahmed, M.J. Biosynthesis, characterization and antimicrobial action of silver nanoparticles from root bark extract of Berberis lycium Royle. Pak. J. Pharm. Sci. 2016, 29, 131–137. [Google Scholar]
- Anzabi, Y. In vitro study of Berberis vulgaris, Actinidia deliciosa and Allium cepa L. antibacterial effects on Listeria monocytogenes. Crescent J. Med. Biol. Sci. 2015, 2, 111–115. [Google Scholar]
- Thakur, P.; Chawla, R.; Narula, A.; Sharma, R.K. Protective effect of Berberis aristata against peritonitis induced by carbapenem-resistant Escherichia coli in a mammalian model. J. Glob. Antimicrob. Resist. 2017, 9, 21–29. [Google Scholar] [CrossRef]
- Shahid, M.; Rahim, T.; Shahzad, A.; Latif, T.A.; Fatma, T.; Rashid, M.; Raza, A.; Mustafa, S. Ethnobotanical studies on Berberis aristata DC. root extracts. African J. Biotechnol. 2009, 8, 556–563. [Google Scholar]
- Rizwan, M.; Nasir, H.; Shah, S.Z. Phytochemical and biological screening of Berberis aristata. Adv. Life Sci. 2017, 57, 1–7. [Google Scholar]
- Amin, A.H.; Subbaiah, T.V.; Abbasi, K.M. Berberine sulfate: Antimicrobial activity, bioassay, and mode of action. Can. J. Microbiol. 1969, 15, 1067–1076. [Google Scholar] [CrossRef]
- Freile, M.L.; Giannini, F.; Pucci, G.; Sturniolo, A.; Rodero, L.; Pucci, O.; Balzareti, V.; Enriz, R.D. Antimicrobial activity of aqueous extracts and of berberine isolated from Berberis heterophylla. Fitoterapia 2003, 74, 702–705. [Google Scholar] [CrossRef]
- Hyun, T.K.; Kim, H.C.; Kim, J.S. In vitro Screening for Antioxidant, Antimicrobial, and Antidiabetic Properties of Some Korean Native Plants on Mt. Halla, Jeju Island. Indian J. Pharm. Sci. 2015, 77, 668–674. [Google Scholar] [PubMed]
- Kosalec, I.; Gregurek, B.; Kremer, D.; Zovko, M.; Sanković, K.; Karlović, K. Croatian barberry (Berberis croatica Horvat): A new source of berberine—Analysis and antimicrobial activity. World J. Microbiol. Biotechnol. 2009, 25, 145–150. [Google Scholar] [CrossRef]
- Malik, T.A.; Kamili, A.N.; Chishti, M.Z.; Ahad, S.; Tantry, M.A.; Hussain, P.R.; Johri, R.K. Breaking the resistance of Escherichia coli: Antimicrobial activity of Berberis lycium Royle. Microb. Pathog. 2017, 102, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, R.; Speciale, A.; Costanzo, R.; Annino, A.; Ragusa, S.; Rapisarda, A.; Pappalardo, M.S.S.; Iauk, L. Berberis aetnensis C. Presl. extracts: Antimicrobial properties and interaction with ciprofloxacin. Int. J. Antimicrob. Agents 2003, 22, 48–53. [Google Scholar] [CrossRef]
- Villinski, J.R.; Dumas, E.R.; Chai, H.B.; Pezzuto, J.M.; Angerhofer, C.K.; Gafner, S. Antibacterial activity and alkaloid content of Berberis thunbergii, Berberis vulgaris and Hydrastis canadensis. Pharm. Biol. 2003, 41, 551–557. [Google Scholar] [CrossRef]
- Bereksi, M.S.; Hassaïne, H.; Bekhechi, C.; Abdelouahid, D.E. Evaluation of Antibacterial Activity of some Medicinal Plants Extracts Commonly Used in Algerian Traditional Medicine against some Pathogenic Bacteria. Pharmacogn. J. 2018, 10, 507–512. [Google Scholar] [CrossRef] [Green Version]
- Maznah, I.; Teoh, S.L.; Loh, P. Determination of total antioxidant activity of selected local medicinal plants. In Proceedings of the Herbs an International Conference and Exhibitions, Mines, Seri Kembangan, Malaysia, 9–11 November 1999; pp. 124–128. [Google Scholar]
- Eddouks, M.; Maghrani, M.; Lemhadri, A.; Ouahidi, M.-L.; Jouad, H. Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the south-east region of Morocco (Tafilalet). J. Ethnopharmacol. 2002, 82, 97–103. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Tuzcu, M.; Borawska, M.H.; Jabłonski, J.; Guler, O.; Sahin, N.; Hayirli, A. Berberis vulgaris root extract alleviates the adverse effects of heat stress via modulating hepatic nuclear transcription factors in quails. Br. J. Nutr. 2013, 110, 609–616. [Google Scholar] [CrossRef]
- Komal, S.; Ranjan, B.; Neelam, C.; Birendra, S.; Kumar, S.N. Berberis Aristata: A Review. Int. J. Res. Ayurveda Pharm. 2011, 2, 383–388. [Google Scholar]
- Luo, A.; Fan, Y. Antioxidant activities of berberine hydrochloride. J. Med. Plants Res. 2011, 5, 3702–3707. [Google Scholar]
- Pyrkosz-Biardzka, K.; Kucharska, A.Z.; Sokół-Łȩtowska, A.; Strugała, P.; Gabrielska, J. A comprehensive study on antioxidant properties of crude extracts from fruits of Berberis vulgaris L., Cornus mas L. and Mahonia aquifolium nutt. Polish J. Food Nutr. Sci. 2014, 64, 91–99. [Google Scholar] [CrossRef]
- Sun, L.L.; Gao, W.; Zhang, M.M.; Li, C.; Wang, A.G.; Su, Y.L.; Ji, T.F. Composition and antioxidant activit y of the anthocyanins of the fruit of berberis heteropoda schrenk. Molecules 2014, 19, 19078–19096. [Google Scholar] [CrossRef] [PubMed]
- Campisi, A.; Acquaviva, R.; Bonfanti, R.; Raciti, G.; Amodeo, A.; Mastrojeni, S.; Ragusa, S.; Iauk, L. Antioxidant Properties of Berberis aetnensis C. Presl (Berberidaceae) Roots Extract and Protective Effects on Astroglial Cell Cultures. Sci. World J. 2014, 2014, 315473. [Google Scholar] [CrossRef] [PubMed]
- Gundogdu, M. Determination of antioxidant capacities and biochemical compounds of Berberis vulgaris L. Fruits. Adv. Environ. Biol. 2013, 7, 344–348. [Google Scholar]
- Zaorsky, N.G.; Churilla, T.M.; Egleston, B.L.; Fisher, S.G.; Ridge, J.A.; Horwitz, E.M.; Meyer, J.E. Causes of death among cancer patients. Ann. Oncol. 2017, 28, 400–407. [Google Scholar] [CrossRef]
- Hanachi, P.; Kua, S.H.; Asmah, R.; Motalleb, G.; Fauziah, O. Cytotoxic Effect of Berberis vulgaris Fruit Extract on the Proliferation of Human Liver Cancer Cell line (HepG2) and Its Antioxidant Properties. Int. J. Cancer Res. 2006, 2, 1–9. [Google Scholar]
- Hoshyar, R.; Mahboob, Z.; Zarban, A. The antioxidant and chemical properties of Berberis vulgaris and its cytotoxic effect on human breast carcinoma cells. Cytotechnology 2016, 68, 1207–1213. [Google Scholar] [CrossRef]
- Abd El-Wahab, A.E.; Ghareeb, D.A.; Sarhan, E.E.M.; Abu-Serie, M.M.; El Demellawy, M.A. In vitro biological assessment of berberis vulgaris and its active constituent, berberine: Antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complement. Altern. Med. 2013, 13, 218. [Google Scholar] [CrossRef]
- El Khalki, L.; Tilaoui, M.; Jaafari, A.; Ait Mouse, H.; Zyad, A. Studies on the Dual Cytotoxicity and Antioxidant Properties of Berberis vulgaris Extracts and Its Main Constituent Berberine. Adv. Pharmacol. Sci. 2018, 2018, 3018498. [Google Scholar] [CrossRef]
- Choi, M.S.; Oh, J.H.; Kim, S.M.; Jung, H.Y.; Yoo, H.S.; Lee, Y.M.; Moon, D.C.; Han, S.B.; Hong, J.T. Berberine inhibits p53-dependent cell growth through induction of apoptosis of prostate cancer cells. Int. J. Oncol. 2009, 34, 1221–1230. [Google Scholar] [Green Version]
- Balakrishna, A.; Kumar, M.H. Evaluation of synergetic anticancer activity of berberine and curcumin on different models of A549, Hep-G2, MCF-7, Jurkat, and K562 cell lines. Biomed Res. Int. 2015, 2015, 354614. [Google Scholar] [CrossRef]
- Ren, K.; Zhang, W.; Wu, G.; Ren, J.; Lu, H.; Li, Z.; Han, X. Synergistic anti-cancer effects of galangin and berberine through apoptosis induction and proliferation inhibition in oesophageal carcinoma cells. Biomed. Pharmacother. 2016, 84, 1748–1759. [Google Scholar] [CrossRef]
- Sengupta, P.; Raman, S.; Chowdhury, R.; Lohitesh, K.; Saini, H.; Mukherjee, S.; Paul, A. Evaluation of Apoptosis and Autophagy Inducing Potential of Berberis aristata, Azadirachta indica, and Their Synergistic Combinations in Parental and Resistant Human Osteosarcoma Cells. Front. Oncol. 2017, 7, 296. [Google Scholar] [CrossRef]
- Fukuda, K.; Hibiya, Y.; Mutoh, M.; Koshiji, M.; Akao, S.; Fujiwara, H. Inhibition by berberine of cyclooxygenase-2 transcriptional activity in human colon cancer cells. J. Ethnopharmacol. 1999, 66, 227–233. [Google Scholar] [CrossRef]
- Safi, S.; Esseily, F.; El Ezzy, M.; Gali-Muhtasib, H.; Esseily, J.; Diab-Assaf, M.; Lampronti, I.; Saab, A. The ethanol fraction from the stem of Berberis libanotica inhibits the viability of adult T cell leukemia. Minerva Biotecnol. 2012, 24, 129–133. [Google Scholar]
- El-Merahbi, R.; Liu, Y.N.; Eid, A.; Daoud, G.; Hosry, L.; Monzer, A.; Mouhieddine, T.H.; Hamade, A.; Najjar, F.; Abou-Kheir, W. Berberis libanotica ehrenb extract shows anti-neoplastic effects on prostate cancer stem/progenitor cells. PLoS ONE 2014, 9, e112453. [Google Scholar] [CrossRef]
- Diab, S.; Ftdanzi, C.; Léger, D.Y.; Ghezali, L.; Millot, M.; Martin, F.; Azar, R.; Esseily, F.; Saab, A.; Sol, V.; et al. Berberis libanotica extract targets NF-$κ$B/COX-2, PI3K/Akt and mitochondrial/caspase signalling to induce human erythroleukemia cell apoptosis. Int. J. Oncol. 2015, 47, 220–230. [Google Scholar] [CrossRef]
- Du, H.P.; Shen, J.K.; Yang, M.; Wang, Y.G.; Yuan, X.G.; Ma, Q.L.; Jin, J. 4-Chlorobenzoyl berbamine induces apoptosis and G2/M cell cycle arrest through the PI3K/Akt and NF-κB signal pathway in lymphoma cells. Oncol. Rep. 2010, 23, 709–716. [Google Scholar]
- Xie, J.; Ma, T.; Gu, Y.; Zhang, X.; Qiu, X.; Zhang, L.; Xu, R.; Yu, Y. Berbamine derivatives: A novel class of compounds for anti-leukemia activity. Eur. J. Med. Chem. 2009, 44, 3293–3298. [Google Scholar] [CrossRef]
- Nam, S.; Xie, J.; Perkins, A.; Ma, Y.; Yang, F.; Wu, J.; Wang, Y.; Zhen Xu, R.; Huang, W.; Horne, D.A.; et al. Novel synthetic derivatives of the natural product berbamine inhibit Jak2/Stat3 signaling and induce apoptosis of human melanoma cells. Mol. Oncol. 2012, 6, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Nam, S.; Brown, C.E.; Zhao, R.; Starr, R.; Horne, D.A.; Malkas, L.H.; Jove, R.; Hickey, R.J. A novel berbamine derivative inhibits cell viability and induces apoptosis in cancer stem-like cells of human glioblastoma, via up-regulation of miRNA-4284 and JNK/AP-1 signaling. PLoS ONE 2014, 9, e94443. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Nam, S.; Zhao, R.; Tian, Y.; Liu, L.; Horne, D.A.; Jove, R. A novel synthetic derivative of the natural product berbamine inhibits cell viability and induces apoptosis of human osteosarcoma cells, associated with activation of JNK/AP-1 signaling. Cancer Biol. Ther. 2013, 14, 1024–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, H.; Luan, J.; Liu, Q.; Yagasaki, K.; Zhang, G. Suppression of human lung cancer cell growth and migration by berbamine. Cytotechnology 2010, 62, 341–348. [Google Scholar] [CrossRef]
- Wu, J.; Yu, D.; Sun, H.; Zhang, Y.; Zhang, W.; Meng, F.; Du, X. Optimizing the extraction of anti-tumor alkaloids from the stem of Berberis amurensis by response surface methodology. Ind. Crops Prod. 2015, 69, 68–75. [Google Scholar] [CrossRef]
- Bavand, R.; Nemati, F. Cytotoxic effect of the root extract of Berberis orthobotrys on hela cell line. IIOAB J. 2016, 7, 204–208. [Google Scholar]
- Engel, N.; Ali, I.; Adamus, A.; Frank, M.; Dad, A.; Ali, S.; Nebe, B.; Atif, M.; Ismail, M.; Langer, P.; et al. Antitumor evaluation of two selected Pakistani plant extracts on human bone and breast cancer cell lines. BMC Complement. Altern. Med. 2016, 16, 1. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, S.U.; Lee, K.R. Bioactivity-guided isolation of cytotoxic triterpenoids from the trunk of Berberis koreana. Bioorganic Med. Chem. Lett. 2010, 20, 1944–1947. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, S.U.; Lee, K.R. Cytotoxic triterpenoids from Berberis koreana. Planta Med. 2012, 78, 86–89. [Google Scholar] [CrossRef]
- Derosa, G.; Romano, D.; D’Angelo, A.; Maffioli, P. Berberis aristata combined with Silybum marianum on lipid profile in patients not tolerating statins at high doses. Atherosclerosis 2015, 239, 87–92. [Google Scholar] [CrossRef]
- Derosa, G.; Romano, D.; D’Angelo, A.; Maffioli, P. Berberis aristata/Silybum marianum fixed combination (Berberol®) effects on lipid profile in dyslipidemic patients intolerant to statins at high dosages: A randomized, placebo-controlled, clinical trial. Phytomedicine 2015, 22, 231–237. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Maffioli, P. The role of a fixed Berberis aristata/Silybum marianum combination in the treatment of type 1 diabetes mellitus. Clin. Nutr. 2016, 35, 1091–1095. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Romano, D.; Maffioli, P. Effects of a Combination of Berberis aristata, Silybum marianum and Monacolin on Lipid Profile in Subjects at Low Cardiovascular Risk; A Double-Blind, Randomized, Placebo-Controlled Trial. Int. J. Mol. Sci. 2017, 18, 343. [Google Scholar]
- Guarino, G.; Strollo, F.; Carbone, L.; Della Corte, T.; Letizia, M.; Marino, G.; Gentile, S. Bioimpedance analysis, metabolic effects and safety of the association Berberis aristata/Bilybum marianum: A 52-week double-blind, placebo-controlled study in obese patients with type 2 diabetes. J. Biol. Regul. Homeost. Agents 2017, 31, 495–502. [Google Scholar]
- Di Pierro, F.; Putignano, P.; Villanova, N.; Montesi, L.; Moscatiello, S.; Marchesini, G. Preliminary study about the possible glycemic clinical advantage in using a fixed combination of Berberis aristata and Silybum marianum standardized extracts versus only Berberis aristata in patients with type 2 diabetes. Clin. Pharmacol. Adv. Appl. 2013, 5, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Zilaee, M.; Kermany, T.; Tavalaee, S.; Salehi, M.; Ghayour-Mobarhan, M.; Ferns, G.A. Barberry treatment reduces serum anti-heat shock protein 27 and 60 antibody titres and high-sensitivity c-reactive protein in patients with metabolic syndrome: A double-blind, randomized placebo-controlled trial. Phytother. Res. 2014, 28, 1211–1215. [Google Scholar] [CrossRef]
- Brenyo, A.; Aktas, M.K. Review of complementary and alternative medical treatment of arrhythmias. Am. J. Cardiol. 2014, 113, 897–903. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.; Tan, Z.R.; Klaassen, C.D.; Zhou, H.H. Repeated administration of berberine inhibits cytochromes P450 in humans. Eur. J. Clin. Pharmacol. 2012, 68, 213–217. [Google Scholar] [CrossRef]
- Chen, C.; Tao, C.; Liu, Z.; Lu, M.; Pan, Q.; Zheng, L.; Li, Q.; Song, Z.; Fichna, J. A Randomized Clinical Trial of Berberine Hydrochloride in Patients with Diarrhea-Predominant Irritable Bowel Syndrome. Phyther. Res. 2015, 29, 1822–1827. [Google Scholar] [CrossRef]
- Fouladi, R.F. Aqueous extract of dried fruit of berberis vulgaris L. in acne vulgaris, a clinical trial. J. Diet. Suppl. 2012, 9, 253–261. [Google Scholar] [CrossRef]
- Shin, K.S.; Choi, H.S.; Zhao, T.T.; Suh, K.H.; Kwon, I.H.; Choi, S.O.; Lee, M.K. Neurotoxic effects of berberine on long-term l-DOPA administration in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Arch. Pharm. Res. 2013, 36, 759–767. [Google Scholar] [CrossRef]
- Kwon, I.H.; Choi, H.S.; Shin, K.S.; Lee, B.K.; Lee, C.K.; Hwang, B.Y.; Lim, S.C.; Lee, M.K. Effects of berberine on 6-hydroxydopamine-induced neurotoxicity in PC12 cells and a rat model of Parkinson’s disease. Neurosci. Lett. 2010, 486, 29–33. [Google Scholar] [CrossRef]
- Qian, C.; Zhu, F. Berberine chloride can ameliorate the spatial memory impairment and increase the expression of interleukin-1beta and inducible nitric oxide synthase in the rat model of Alzheimer’s disease. BioMed 2006, 3, 1–9. [Google Scholar]
- Ahmed, T.; Gilani, A.U.H.; Abdollahi, M.; Daglia, M.; Nabavi, S.F.; Nabavi, S.M. Berberine and neurodegeneration: A review of literature. Pharmacol. Rep. 2015, 67, 970–979. [Google Scholar] [CrossRef]



| Chemical structure | Name | Plant |
|---|---|---|
 | palmatine | B. vulgaris |
 | berberine | B. vulgaris |
 | oxyberberine | B. vulgaris |
 | isocoridine | B. vulgaris |
 | lambertine | B. vulgaris |
 | magniflorine | B. vulgaris |
 | oxycanthine | B. vulgaris |
 | berbamine | B. aristata |
 | (+)-N-methylcoclaurine | B. montana |
 | (−)-pronuciferine | B. montana |
 | (+)-9-hydroxynuciferine | B. montana |
 | (+)-orientine | B. montana |
 | 2-norberbamunine | B. stoloniferais |
 | berbamunine | B. stoloniferais |
 | aromoline | B. stoloniferais |
 | isotetrandrine | B. stoloniferais |
 | jatrorrhizine | B. umbellate |
| S. No. | Species | Part | Country | Extract/Model/Compound | Tested Micro-Organism | Results | Reference |
|---|---|---|---|---|---|---|---|
| 1 | B. aristata | Stem and leaves | Nepal | Hexane, Ethyl acetate, Methanol | Staphylococcus aureus, Kleibsella pneumoniae, Salmonella typhimurium | Against S. aureus: methanol significant zone of inhibition (21 mm), ethyl acetate extracts moderate activity, hexane extract of stem slightly active. | [73] |
| 2 | B. aristata, and B. ligulata | Bark stem Leaves | Nepal | Ethanol | Bacillus subtilis, Escherichia coli, Pseudomona aeruginosa, Salmonella. typhi, Salmonella dyjenteriae, Salmonella cholerae | Ethanol extract of B. aristata: largest zone of inhibition (21 mm) against B. subtilis and the smallest MBC value (90 mg/mL) for S. aureus. Gram positive bacteria more susceptible to the ethanol extract. B. aristata relatively broad-spectrum antibacterial activity. | [74] |
| 3 | B. vulgaris | Stem | Iran | Ethanol | P. aeruginosa, Acinetobacter baumannii, E. coli and Salmonella enteritidis | MIC determination: stem extracts inhibit the growth of all the studied bacteria (3900 to 37,500 μg/mL) by synergistic effects with ciprofloxacin. | [75] |
| 4 | B. asiatica | Leaves | Uttarakhand, India | Methanol | E. coli, Enterobacter aerogenes, Proteus vulgaris, P. aeruginosa, K. pneumoniae, B. subtilis, S. aureus | Methanol extracts of leaves: high inhibitory potential on S. aureus, K. pneumoniae, E. coli, B. subtilis and P. vulgaris in all concentration. | [76] |
| 5 | B. aristata, B. asiatica, B. lycium | Stem | Bangalore, India | Methanol | Nocardia sp., S. aureus, S. pneumonia, P. aeruginosa, Streptococcus viridians, E. coli | Sensitivity to Nocardia sp., S. pneumonia and E. coli. | [77] |
| 6 | B. glaucocarpa | Root wood | Pakistan | Ethanol | SMRSA, EMRSA, Mycobacterium marinum, E. coli, Trypanosoma brucei | Berberine (MIC = 12.5 and 25 μg/mL), berberine chloroform (MIC = 25 and 12.5 μg/mL) and syringaresinol (12.5 μg/mL): very active against SMRSA, M. marinum and T. brucei. | [78] |
| 7 | B. vulgaris | Stem bark | Romania | Ethanol | Botrytis cinerea | B. vulgaris bark extract, berberine, and fluconazole significantly inhibited growth of B. cinerea. | [79] |
| 8 | B. vulgaris | Ethanol | S. aureus, Staphylococcus epidermidis, K. pneumoniae, B. subtilis, E. coli, Aspergillus niger, Trichoderma, Alternaria solanai | 20 mm zone of inhibition against E. coli. Good activity against B. Subtilis, moderate against Trichoderma, insignificant against other stains. | [80] | ||
| 9 | B. vulgaris and its active constituent, berberine | Root | Egypt | Ethanolic extract | Candida albicans, E. coli | Berberis ethanolic extract and berberine standard can inhibit C. albicans and E. coli growth. | [81] |
| 10 | B. vulgaris | Fruit | Pakistan | Distilled water | S. aureus, Proteus, S. typhi, Salmonella paratyphi A, Salmonella paratyphi B, K. pneumoniae, E. coli, P. aeruginosa | Antibacterial activity against all tested pathogens. | [82] |
| 11 | B. thunbergii | Fruit | Hungary | Juice; water extract and -methanol extract | B. subtilis, Bacillus cereus var. mycoides, E. coli, Serratia marcescens | Juice, water extract and methanol extract showed activity against all bacteria. | [83] |
| 12 | B. calliobotrys | Stems and branches | Pakistan | Methanol | B. subtilis, P. aeruginosa, S. aureus fungal strains namely C. albicans, Penicillium notatum | The methanol extract, ethyl acetate and n-butanol fractions: maximum zone of inhibition against all bacterial strains especially S. aureus and antifungal effects. | [84] |
| 13 | B. lycium | Roots | Libya | Distilled water, ethanol, isopropanol and methanol | Pseudomonas sp., E. coli, Streptococcus sp., Staphylococcus sp. | Methanolic displayed maximum inhibitory zone (16 mm), isopropanol extract (13 mm) and ethanol extract (12 mm). The aqueous extract exhibited the least inhibitory zone (10 mm). The methanolic extract: maximum inhibitory zone (12 mm), Pseudomonas (11 mm) and Staphylococcus (10 mm). | [85] |
| 14 | B. hispanica | Root Bark | Marocco | Ethanolic extract | Mycobactérium smegmatis, Mycobacterium aurum | The ethanolic extract from root bark displayed an important antimycobacterial activity. The inhibition zones for M. aurum A+ were significantly larger than those for M. smegmatis MC2. | [86] |
| 15 | B. ruscifolia | - | Argentina | Acetone, chloroform-methanol (1:1) and methanol | E. coli, P. aeruginosa, Listeria monocytogenes, S. aureus | All extracts exhibited antibacterial activity with MIC varying from 16 to 2 mg/mL. The highest inhibition with acetonic and chloroform-methanolic extracts of species against S. aureus (MIC = 2 mg/mL). Methanolic extracts B. ruscifolia showed no antibacterial activity against all tested bacteria. | [87] |
| 16 | B. aristata | Stem bark | India | Ethanol and aqueous extracts | Shigella flexneri, Shigella sonnei, Shigella dysenteriae, Shigella boydii | Extracts of B. aristata: antibacterial activity against four strains of Shigella (8 and 23 mm). | [88] |
| 17 | B. aristata, B. asiatica, B. chitria and B. lycium | Root and stem | India | Ethanol | Micrococcus luteus, B. subtilis, B. cereus, Enterobacter aerogenus, E. coli, K. pneumoniae, Proteus mirabilis, P. aeruginosa, S. aureus, S. typhimurium, Streptococcus pneumonia, Fungal strains Aspergillus nidulans, C. albicans, Aspergillus terreus, Trichophyton rubrum, Cistus albidus, Aspergillus flavus, A. niger | B. lycium, B. aristata and B. asiatica root extract showed significant antifungal activity against A. terreus and A. flavus. B. aristata root and B. lycium (stem) extracts gave very low MIC values (0.31 μg/mL) as compared to other tested species. | [89] |
| 18 | B. Lycium | Root | Pakistan | Ethanol, petroleum ether | S. aureus, S. epidermidis, B. subtilis, S. typhi, E. coli, C. albicans | The ethanolic and aqueous crud root extract: most effective antifungal and antibacterial agents. | [90] |
| 19 | B. integerrima Syn: B. densiflora | Roots | Iran | Methanol | Brucella abortus | MIC and MBC results, jatrorhizine exhibited higher antibacterial activity with MIC (0.78 μg/mL) and MBC (1.56 μg/mL) compared with the standard (streptomycin, 10 μg/mL). | [91] |
| 20 | B. lycium | Roots | Pakistan | Hydric extract | E. coli, Pseudomonas, Staphylococcus, Proteus | Significant activity against E. coli and Proteus (80 to 100%), while it demonstrated a good activity against Pseudomonas and Staphylococcus (60 to 70%). | [92] |
| 21 | B. aristata | Bark and leaves | India | Methanol, ethanol and hexane | B. subtilis, Agrobacterium tumefaciens, E. coli, Xanthomonas. Phaseoli, Erwinia chrysanthemi | All the extracts of tested plants showed variable activity against all the tested bacterial strains. Methanol extract revealed highest antibacterial activity (11 mm) recorded against E. chrysanthemi. Hexane extract: totally inactive against all the tested strains. | [93] |
| 22 | B. aristata | Roots | India | Aqueous and alcohol extracts | S. aureus, B. subtilis, E. coli, S. typhimurium | Alcoholic and aqueous extract showed antimicrobial activity against four tested bacteria. B. aristata exhibited highest zone of inhibition for B. subtilis followed by S. aureus, E. coli and S. typhimurium. | [94] |
| 23 | B. microphylla | Leaves, stems and roots | Chile | Methanol | E. coli, S. typhimurium, L. monocytogenes, E. aerogenes, S. aureus, B. cereus, S. epidermidis and B. subtilis | All extract possesses significant antibacterial activity against Gram-positive bacteria but not against Gram-negative bacteria. | [95] |
| 24 | B. lycium | Root bark | Pakistan | E. coli, K. pneumoniae, P. aeruginosa, S. aureus, B. subtilis | Silver nanoparticles were very active against Gram-negative and Gram-positive bacteria Aqueous bark extract (10 μg/mL) possess highest activity against E. coli and P. aeruginosa. | [96] | |
| 25 | B. vulgaris | Fruit | Iran | L. monocytogenes | Average diagonal of growing area in disk diffusion test for species: 12 mm and MIC was 125 μg/mL and MBC of B. vulgaris was 500 μg/mL. | [97] | |
| 26 | B. aristata | Stem bark | Alcohol | In vivo in an animal model using Sprague Dawley rats | Carbapenem-resistant E. coli | An aquo-alcoholic extract of the species: effectively manage peritonitis induced by Carbapenem-resistant E. coli in a rat model at a single post-exposure prophylactic dose of 0.5 mg/kg body weight. | [98] |
| 27 | B. aristata | Roots | India | Aqueous and alcoholic extract of fresh roots, as well as aqueous extract of dried roots | S. aureus, S. epidermidis, Streptococcus pyogenes, Streptococcus viridans, Enterococcus faecalis, B. subtilis, B. cereus, E. coli, K. pneumoniae, P. aeruginosa, P. vulgaris, P. mirabilis, S. typhi, S. paratyphi A, S. typhimurium, S. dysenteriae type 1, Vibrio cholerae | All three extracts displayed wide antibacterial activity against Gram-positive bacteria. Among the Gram-negative bacteria tested, the antibacterial activity was limited to E. coli, S. typhimurium, S. dysenteriae type 1 and V. cholerae. All extracts also possess antifungal activity against the fungal species tested, except Candida krusei. | [99] |
| 28 | B. aristata | Root Stem Leaf | Pakistan | E. coli, S. typhi, S. aureus, Shigella, Citrobacter, P. vulgaris,Enterobacter, Streptococcus pyrogenes, V. cholera, Klebsiella spp., A. niger, Cladosporium, Rhizoctonia, Alternaria, Trichoderma, Penicillium, Curvularia, Paecilomyces and Rhizopus | The extracts significantly inhibited the growth of the studied microbes, except A. niger, Curvularia, Paecilomyces and Rhizopus. | [100] | |
| 29 | B. aristata | India | V. cholerae, S. aureus | All the strains of V. cholerae are susceptible. All the Salmonella sp., Pseudomonas sp., and some of the E. coli strains are highly resistant, except some strains of E. coli as AL26, and Shigella sp. are susceptible. All Xanthomonas sp. were highly susceptible. Berberine sulfate showed antifungal action against C. albicans, Candida tropicalis, Trichophyton mentagrophytes, Microsporum gypseum, Cryptococcus neoformans and Sporothrix schenkii, Mycobacterium tuberculosis var. hominis H37RV and Entamoeba histolytica. | [101] | ||
| 30 | B. heterophylla | Leaves, stems and roots berberine | Argentina | S. aureus, E. faecali, P.aeruginosa, E. coli, C. albicans, Candida glabrata, Candida haemulonii, Candida lusitaniae, C. krusei, Candida parapsilosis | The aqueous extracts of B. heterophylla do not possess significant antimicrobial activity. Berberine displayed a significant antibacterial and antifungal activity against S. aureus and different Candida spp., some of them obtained from the clinical isolated. | [102] | |
| 31 | B. amurensis | Branches and leaves | Korea | Bacillus atrophaeus, Kocuria rhizophila, M. luteus, S. epidermidis, B. subtilis subsp. Spizizenii, K. pneumoniae, Enterobacter cloacae, Salmonella enterica subsp. enterica, P. aeruginosa | No significant activity against gram-negative bacteria. | [103] | |
| 32 | B. croatica and B. vulgaris | Roots, leaves, and twigs | Croatia | Ethanol | B. subtilis, S. aureus, E. coli, P. aeruginosa, C. albicans | Extracts of both species: significant antibacterial activity against the Gram-positive bacteria. Root extracts of B. croatica: activity against P. aeruginosa, and leaf extracts against B. subtilis. Neither species possessed antifungal activity. Leaf extracts of B. croatica: antibacterial activity against B. subtilis. Likewise, neither of the species extracts showed activity against E. coli and C. albicans, except when were diluted. Ethanolic extracts of twigs of both species: inactive against B. subtilis and against S. aureus, with the exception of B. croatica twig from Kiza locality. | [104] |
| 33 | B. lycium | Roots | India | Hexane extract, Methanolic extract, aqueous extract and berberine | K. pneumonia, E. coli, P. aeuroginosa, S. aureus, B. subtilis, C. albicans, A. niger, Aspergillus fumigates | Methanolic extract of species was highly effective against E. coli, S. aureus, B. subtilis, C. albicans, A. fumigates. Pure berberine was effective against E. coli and C. albicans. | [105] |
| 34 | B. aetnensis | Roots | Italy | Ethanol ether and chloroform | S. aureus, B. subtilis, E. faecalis, E. coli, P. aeruginosa, Stenotrophomonas maltophilia, against 14 strains of nosocomial origin: two strains of S. aureus (1 Met-S, 1 Met-R); four strains of S. epidermidis (2 Met-S, 2 Met-R); three strains of E. coli; four strains of P. aeruginosa, Hafnia alvei and C. albicans, C. parapsilosis, C. krusei | The root and leaf extracts showed a greater activity against Gram-positive bacteria and yeasts than against Gram-negative bacteria, except for P. aeruginosa. The chloroform extract of leaves was more active than the ethanol. | [106] |
| 35 | B. thunbergii, B. vulgaris | Roots | USA | E. coli, P. aeruginosa, S. aureus, S. mutans, and S. pyogenes | Ethanolic extracts more active against studied bacteria, strongest activity against S. pyogenes and S. aureus. | [107] | |
| 36 | B. vulgaris | Root bark | Algeria | Methanol and water | S. aureus, E. faecalis, E. coli, E. cloacae, K. pneumoniae, P. aeruginosa | The extracts of species root barks presented a strong activity against S. aureus (23.0 mm), a weak activity against E. faecalis (13.0 mm) and no activity toward other strains. | [108] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehi, B.; Selamoglu, Z.; Sener, B.; Kilic, M.; Kumar Jugran, A.; de Tommasi, N.; Sinisgalli, C.; Milella, L.; Rajkovic, J.; Flaviana B. Morais-Braga, M.; et al. Berberis Plants—Drifting from Farm to Food Applications, Phytotherapy, and Phytopharmacology. Foods 2019, 8, 522. https://doi.org/10.3390/foods8100522
Salehi B, Selamoglu Z, Sener B, Kilic M, Kumar Jugran A, de Tommasi N, Sinisgalli C, Milella L, Rajkovic J, Flaviana B. Morais-Braga M, et al. Berberis Plants—Drifting from Farm to Food Applications, Phytotherapy, and Phytopharmacology. Foods. 2019; 8(10):522. https://doi.org/10.3390/foods8100522
Chicago/Turabian StyleSalehi, Bahare, Zeliha Selamoglu, Bilge Sener, Mehtap Kilic, Arun Kumar Jugran, Nunziatina de Tommasi, Chiara Sinisgalli, Luigi Milella, Jovana Rajkovic, Maria Flaviana B. Morais-Braga, and et al. 2019. "Berberis Plants—Drifting from Farm to Food Applications, Phytotherapy, and Phytopharmacology" Foods 8, no. 10: 522. https://doi.org/10.3390/foods8100522