Effects of Pulsed Electric Field-Assisted Osmotic Dehydration and Edible Coating on the Recovery of Anthocyanins from In Vitro Digested Berries
Abstract
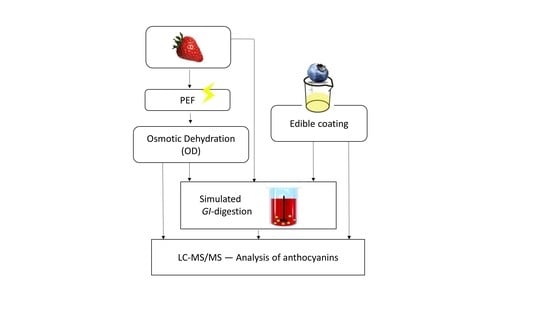
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Raw Materials
2.3. Strawberry Samples—Pulsed Electric Field (PEF) Treatment
2.4. Strawberry Samples—Osmotic Dehydration (OD) Treatment
2.5. Blueberry Samples—Edible Coating
2.6. In Vitro Digestion of Berry Samples
2.7. Extraction of Anthocyanins
2.8. Determination of Anthocyanins using LC-MS/MS
2.9. Recovery of Anthocyanins
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effect of PEF-Assisted OD on the Recovery of Anthocyanins in Strawberry
3.2. Effect of Edible Coating on the Recovery of Anthocyanins in Blueberry Samples
3.3. Recovery of Anthocyanins from Processed Strawberry after In Vitro Digestion
3.4. Recovery of Anthocyanins from Coated Blueberry after In Vitro Digestion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, B.; Kortesniemi, M. Clinical evidence on potential health benefits of berries. Curr. Opin. Food Sci. 2015, 2, 36–42. [Google Scholar] [CrossRef]
- Heneghan, C.; Kiely, M.; Lyons, J.; Lucey, A. The effect of berry-based food interventions on markers of cardiovascular and metabolic health: A systematic review of randomized controlled trials. Mol. Nutr. Food Res. 2018, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; Snadhya, K.; Ellis, C.L.; Tadapaneni, R.; Kappagoda, C.T.; Burton-Freeman, B.M. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br. J. Nutr. 2011, 106, 913–922. [Google Scholar] [CrossRef]
- Correa-Betanzo, J.; Allen-Vercoe, E.; McDonald, J.; Schroeler, K.; Corredig, M.; Paliyath, G. Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem. 2014, 165, 522–531. [Google Scholar] [CrossRef]
- Kay, C.D. Aspects of anthocyanin absorption, metabolism and pharmacokinetics in humans. Nutr. Res. Rev. 2006, 19, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.S.; Wang, L.H.; Bekhit, A.E.A.; Yang, J.; Hou, Z.P.; Wang, Y.Z.; Dai, Q.Z.; Zeng, X.A. A review of sublethal effects of pulsed electric field on cells in food processing. J. Food Eng. 2018, 223, 32–41. [Google Scholar] [CrossRef]
- Angersbach, A.; Heinz, V.; Knorr, D. Effects of pulsed electric fields on cell membranes in real food systems. Innov. Food Sci. Emerg. Technol. 2000, 1, 135–149. [Google Scholar] [CrossRef]
- Tylewicz, U.; Tappi, S.; Mannozzi, C.; Romani, S.; Dellarosa, N.; Laghi, L.; Ragni, L.; Rocculi, P.; Dalla Rosa, M. Effect of pulsed electric field (PEF) pre-treatment coupled with osmotic dehydration on physico-chemical characteristics of organic strawberries. J. Food Eng. 2017, 213, 2–9. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Tylewicz, U.; Castro-Giraldez, M.; Fito, P.J.; Ragni, L.; Dalla Rosa, M. Effect of pulsed electric fields pre-treatment on mass transport during the osmotic dehydration of organic kiwifruit. Innov. Food Sci. Emerg. Technol. 2016, 38, 243–251. [Google Scholar] [CrossRef]
- Ahmed, I.; Qazi, I.M.; Jamal, S. Developments in osmotic dehydration technique for the preservation of fruits and vegetables. Innov. Food Sci. Emerg. Technol. 2016, 34, 29–43. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, S.V. Osmotic dehydration of fruits and vegetables—A review. J. Food Sci. Technol. 2014, 51, 1654–1673. [Google Scholar] [CrossRef] [PubMed]
- Mannozzi, C.; Cecchini, J.; Tylewicz, U.; Siroli, L.; Patrignani, F.; Lanciotti, R.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT Food Sci. Technol. 2016, 85, 440–444. [Google Scholar] [CrossRef]
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by aplication of different edible coatings: A review. LWT Food Sci. Technol. 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Vieira, J.M.; Flores-López, M.L.; Rodríguez, D.J.; Souza, M.C.; Vicente, A.A.; Martins, J.T. Effect of chitosan-Aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef]
- Mannozzi, C.; Tylewicz, F.C.; Siroli, L.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Effects of chitosan based coating enriched with procyanidin by-products on quality of fresh blueberries during storage. Food Chem. 2018, 251, 18–24. [Google Scholar] [CrossRef]
- Escamilla-García, M.; Rodriguez-Hernández, M.J.; Hernández-Hernández, H.M.; Delgado-Sánchez, L.; García-Almendárez, B.E.; Amaro-Reyes, A.; Regalado-González, C. Effect of an edible coating based on chitosan and oxidized starch on shelf life of Carica papaya L., and its physicochemical and antimicrobial properties. Coatings 2018, 8, 318. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Li, D.; Ho, C.T.; Li, J.; Wan, X. The absorption, distribution, metabolism and excretion of procyanidins. Food Funct. 2016, 7, 1273–1281. [Google Scholar] [CrossRef]
- Dellarosa, N.; Tappi, S.; Ragni, L.; Laghi, L.; Rocculi, P.; Dalla Rosa, M. Metabolic response of fresh-cut apples induced by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2016, 38, 356–364. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, C.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardized static in vitro digestion method suitable for food—An international consensus. Food Nutr. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Bunea, A.; Ruginǎ, D.; Sconţa, Z.; Pop, R.M.; Pintea, A.; Socaciu, C.; Tǎbǎrǎn, F.; Grootaert, C.; Struijs, K.; VanCamp, J. Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in B16-F10 metastatic murine melanoma cells. Phytochemistry 2013, 95, 436–444. [Google Scholar] [CrossRef]
- You, Q.; Wang, B.; Chen, F.; Huang, Z.; Wang, X.; Luo, P.G. Comparison of anthocyanins and phenolics in organically and conventionally grown blueberries in selected cultivars. Food Chem. 2011, 125, 201–208. [Google Scholar] [CrossRef]
- Silva, F.L.; Escribano-Bailón, M.T.; Alonso, J.J.P.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanin pigments in strawberry. LWT Food Sci. Technol. 2007, 40, 374–382. [Google Scholar] [CrossRef]
- Buendía, B.; Gil, M.I.; Tudela, J.A.; Gady, A.L.; Medina, J.J.; Soria, C.; López, J.M.; Tomás-Barberán, F.A. HPLC-MS analysis of proanthocyanidin oligomers and other phenolics in 15 strawberry cultivars. J. Agric. Food Chem. 2010, 58, 3916–3926. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Dodds, E.Y.; Leong, S.Y.; Eyres, G.T.; Burritt, D.J.; Oey, I. Effect of pulsed electric fields on the structure and frying quality of “kumara” sweet potato tubers. Innov. Food Sci. Emerg. Technol. 2017, 39, 197–208. [Google Scholar] [CrossRef]
- Ade-Omowaye, B.; Talens, P.; Angersbach, A.; Knorr, D. Kinetics of osmotic dehydration of red bell peppers as influenced by pulsed electric field pretreatment. Food. Res. Int. 2003, 36, 475–483. [Google Scholar] [CrossRef]
- Gibson, L.; Rupasinghe, H.P.V.; Forney, C.F.; Eaton, L. Characterization of changes in polyphenols, antioxidant capacity and physico-chemical parameters during lowbush blueberry fruit ripening. Antioxidants 2013, 2, 216–229. [Google Scholar] [CrossRef]
- González, B.; Vogel, H.; Razmilic, I.; Wolfram, E. Polyphenol, anthocyanin and antioxidant content in different parts of Maqui fruits (Aristotelia chilensis) during ripening and conservation treatments after harvest. Ind. Crop. Prod. 2015, 76, 158–165. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, H.; He, S.; Lou, Q.; Yu, M.; Tang, M. metabolism and prebiotics activity of anthocyanins from black rice (Oryza sativa L.) in vitro. PLoS ONE 2018, 13, e0195754. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell 2018, 9, 416–431. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Yuan, C.; Wang, H.; Liu, Y.; Wang, L.; Liu, Y. Stability of anthocyanins and their degradation products from Cabernet Sauvignon red wine under gastrointestinal pH and temperature conditions. Molecules 2018, 23, 354. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Conte, A. The first tract of alimentary canal as an extractor. Release of phytochemicals from solid food matrices during simulated digestion. J. Food Biochem. 2011, 36, 555–568. [Google Scholar] [CrossRef]
- Kamiloghu, S.; Pasli, A.A.; Ozcelik, B.; Camp, J.V.; Capanoglu, E. Colour retention, anthocyanin stability and antioxidant capacity in black carrot (Daucus carota) jams and marmalades: Effects of processing, storage conditions and in vitro gastrointestinal digestion. J. Funct. Foods 2015, 13, 1–10. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.J.; Tomas-Barberán, F.A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Chapman, D. Preservation of membranes in anhydrobiotic organisms—The role of trehalose. Science 1984, 223, 701–703. [Google Scholar] [CrossRef]
- Kopjar, M.; Jakšić, K.; Piližota, V. Influence of sugars and chlorogenic acid addition on anthocyanin content, antioxidant activity and color of blackberry juice during storage. J. Food Process. Preserv. 2012, 36, 545–552. [Google Scholar] [CrossRef]
- Lončarić, A.; Pichler, A.; Trtinjak, I.; Piližota, V.; Kopjar, M. Phenolics and antioxidant activity of freeze-dried sour cherry puree with addition of disaccharides. LWT Food Sci. Technol. 2016, 73, 391–396. [Google Scholar] [CrossRef]
- Tang, M.; Waring, A.J.; Hong, M. Trehalose-Protected lipid membranes for determining membrane protein structure and insertion. J. Magn. Reson. 2007, 184, 222–227. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Patist, A.; Zoerb, H. Preservation mechanisms of trehalose in food and biosystems. Colloids Surf. B Biointerfaces 2004, 40, 107–113. [Google Scholar] [CrossRef]
- Koide, S.S. Chitin-Chitosan: Properties, benefits and risks. Nutr. Res. 1998, 18, 1091–1101. [Google Scholar] [CrossRef]
- Resende, S.; Goncalves, G.A.S.; Reis, K.C.; Tonoli, H.D.; Boas, E.V.B.V. Chitosan/cellulose nanofibril nanocomposite and its effect on quality of coated strawberries. J. Food Qual. 2018, 2018. [Google Scholar] [CrossRef]
- He, B.; Ge, J.; Yue, P.; Yue, X.; Fu, R.; Liang, J.; Gao, X. Loading of anthocyanins on chitosan nanoparticles influences anthocyanin degradation in gastrointestinal fluids and stability in beverage. Food Chem. 2017, 221, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Hackman, R.; Polagruto, J.A.; Zhu, Q.Y.; Sun, B.; Fujii, H.; Keen, C.L. Flavanols: Digestion, absorption and bioactivity. Phytochem. Rev. 2008, 7, 195–208. [Google Scholar] [CrossRef]
- Serra, A.; Macià, A.; Romero, M.P.; Valls, J.; Bladé, C.; Arola, L.; Motilva, M.J. Bioavailability of procyanidins dimers and trimers and matrix food effects in in vitro and in vivo models. Br. J. Nutr. 2010, 103, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Lee, R.; Scheuller, H.S.; Heber, D. Identification of phenolic compounds in strawberries by liquid chromatography electrospray ionization mass spectroscopy. Food Chem. 2006, 97, 1–11. [Google Scholar] [CrossRef] [Green Version]



| Anthocyanin | Retention Time (min.) | MRM Transitions (m/z) [M + H]+ | Collision Energy (V) |
|---|---|---|---|
| cyanidin-3,5-di-O-glucoside | 1.3 | 611.00 → 287.05 | 37 |
| malvidin-3,5-diglucoside | 2.4 | 655.10 → 331.00 | 35 |
| malvidin-3-O-glucoside | 4.7 | 493.00 → 331.00 | 33 |
| petunidin-3-O-glucoside | 3.5 | 478.90 → 317.05 | 23 |
| cyanidin-3-O-arabinoside | 3.0 | 418.90 → 287.00 | 20 |
| peonidin-3-O-glucoside | 3.3 | 462.90 → 301.00 | 22 |
| delphinidin-3-O-glucoside | 2.1 | 465.20 → 303.00 | 22 |
| cyanidin-3-O-glucoside | 2.8 | 448.90 → 287.12 | 23 |
| Sample | Anthocyanins mg·Kg−1 DW | |||
|---|---|---|---|---|
| Cyanidin-3-O-Glucoside | Petunidin-3-O-Glucoside | Cyanidin-3-O-Arabinoside | Peonidin-3-O-Glucoside | |
| Untreated | 91.75 ± 5.48 b | 0.61 ± 0.01 a | 0.037 ± 0.001 bc | 0.36 ± 0.02 bc |
| PEF_100 | 90.50 ± 5.51 b | 0.37 ± 0.02 d | 0.026 ± 0.001 cde | 0.41 ± 0.03 b |
| PEF_200 | 107.15 ± 4.16 a | 0.56 ± 0.03 ab | 0.054 ± 0.004 a | 0.55 ± 0.01 a |
| OD_S | 81.99 ± 4.33b c | 0.33 ± 0.01 d | 0.037 ± 0.003 bc | 0.34 ± 0.01 bcd |
| OD_T | 66.56 ± 5.20 de | 0.47 ± 0.01 c | 0.057 ± 0.005 a | 0.37 ± 0.01 b |
| PEF_100 + OD_S | 63.76 ± 0.67 e | 0.35 ± 0.04 d | 0.020 ± 0.003 de | 0.37 ± 0.03 b |
| PEF_200 + OD_S | 73.51 ± 1.89 cd | 0.38 ± 0.02 d | 0.031 ± 0.002 bcd | 0.27 ± 0.01 d |
| PEF_100 + OD_T | 79.14 ± 1.80 c | 0.38 ± 0.01 d | 0.039 ± 0.001 b | 0.39 ± 0.02 b |
| PEF_200 + OD_T | 75.17 ± 4.54 cd | 0.35 ± 0.02 d | 0.016 ± 0.001 e | 0.30 ± 0.01 cd |
| Sample | Anthocyanins mg·Kg−1 DW | ||
|---|---|---|---|
| Cyanidin-3-O-Glucoside | Malvidin-3-O-Glucoside | Petunidin-3-O-Glucoside | |
| FT0 | 285.06 ± 14.41 c | 3115.57 ± 6.72 b | 1608.55 ± 103.11 d |
| FT14 | 345.98 ± 14.65 ab | 3397.17 ± 97.75 a | 1897.46 ± 24.70 bc |
| CT0 | 210.87 ± 10.78 d | 2519.57 ± 99.88 c | 1223.83 ± 46.11 e |
| CT14 | 355.21 ± 17.17 a | 3529.05 ± 18.77 a | 2121.66 ± 58.58 ab |
| CpT0 | 297.71 ± 9.56 c | 2528.33 ± 112.29 c | 1685.97 ± 77.91 cd |
| CpT14 | 310.04 ± 6.47b c | 3547.34 ± 84.20 a | 2237.28 ± 107.31 a |
| Cyanidin-3-O-Arabinoside | Peonidin-3-O-Glucoside | Delphinidin-3-O-Galactoside | |
| FT0 | 163.25 ± 4.62 b | 144.27 ± 1.20 c | 1715.19 ± 21.22 c |
| FT14 | 198.91 ± 1.70 a | 171.49 ± 3.97 b | 2134.96 ± 98.39 ab |
| CT0 | 167.62 ± 9.92 b | 114.45 ± 3.36 d | 1389.58 ± 1.96 d |
| CT14 | 194.50 ± 0.31 a | 163.77 ± 1.12 b | 2313.76 ± 118.86 a |
| CpT0 | 159.81 ± 11.08 b | 122.31 ± 3.68 d | 2020.33 ± 10.24 b |
| CpT14 | 197.04 ± 3.99 a | 193.75 ± 9.61 a | 2117.93 ± 54.36 ab |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, G.; Tylewicz, U.; Dalla Rosa, M.; Andlid, T.; Alminger, M. Effects of Pulsed Electric Field-Assisted Osmotic Dehydration and Edible Coating on the Recovery of Anthocyanins from In Vitro Digested Berries. Foods 2019, 8, 505. https://doi.org/10.3390/foods8100505
Oliveira G, Tylewicz U, Dalla Rosa M, Andlid T, Alminger M. Effects of Pulsed Electric Field-Assisted Osmotic Dehydration and Edible Coating on the Recovery of Anthocyanins from In Vitro Digested Berries. Foods. 2019; 8(10):505. https://doi.org/10.3390/foods8100505
Chicago/Turabian StyleOliveira, Gabriel, Urszula Tylewicz, Marco Dalla Rosa, Thomas Andlid, and Marie Alminger. 2019. "Effects of Pulsed Electric Field-Assisted Osmotic Dehydration and Edible Coating on the Recovery of Anthocyanins from In Vitro Digested Berries" Foods 8, no. 10: 505. https://doi.org/10.3390/foods8100505