An Apple and Acáchul Berry Snack Rich in Bioaccessible Antioxidants and Folic Acid: A Healthy Alternative for Prenatal Diets
Abstract
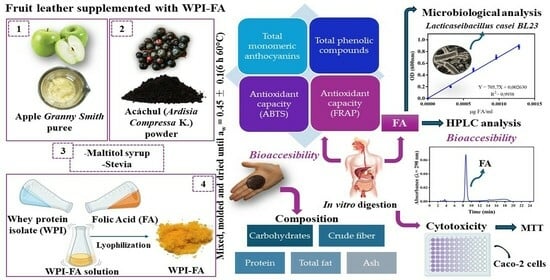
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Composition Characterization of the Supplemented Fruit Leather
2.2.2. Moisture and Water Activity (aw)
2.2.3. In Vitro Gastrointestinal Digestion
2.2.4. Determination of Antioxidant Capacity and Bioactive Compounds
Preparation of Extracts
Total Phenolic Compounds (TPCs)
Antioxidant Capacity (AC)
- Radical Discoloration Method (ABTS•+)
- Antioxidant Iron Reducing Power (FRAP)
Total Monomeric Anthocyanins (ACY)
2.2.5. Folic Acid (FA)
2.2.6. Biological Studies: Cytotoxicity
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Composition Characterization
3.2. Bioaccessibility of Bioactive Compounds and Antioxidant Capacity
3.3. FA Quantification
Bioaccessibility of FA
3.4. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kancherla, V.; Botto, L.; Rowe, L.; Shlobin, N.; Caceres, A.; Arynchyna-Smith, A.; Zimmerman, K.; Blount, J.; Kibruyisfaw, Z.; Ghotme, K.; et al. Preventing birth defects, saving lives, and promoting health equity: An urgent call to action for universal mandatory food fortification with folic acid. Lancet Glob. Health 2022, 10, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Marchetta, C.M.; Devine, O.J.; Crider, K.S.; Tsang, B.L.; Cordero, A.M.; Guo, J.; Berry, R.J.; Rosenthal, J.; Mulinare, J.; Mersereau, P.; et al. Assessing the association between natural food folate intake and blood folate concentrations: A systematic review and Bayesian meta-analysis of trials and observational studies. Nutrients 2015, 7, 2663–2686. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Congenital Disorders. 2023. Available online: https://www.who.int/es/news-room/fact-sheets/detail/birth-defects (accessed on 10 July 2023).
- World Health Organization. Healthy Diet. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 8 August 2021).
- Ŝeremet, D.; Mandura, A.; Vojvodić, A.; Martinić, A.; Galić, K.; Komes, D. Challenges in confectionery industry: Development and storage stability of innovative white tea-based candies. J. Food Sci. 2020, 85, 2060–2068. [Google Scholar] [CrossRef] [PubMed]
- Góngora-Salazar, V.A.; Vázquez-Encalada, S.; Corona-Cruz, A.; Segura-Campos, M.R. Stevia rebaudiana: A sweetener and potential bioactive ingredient in the development of functional cookies. J. Funct. Foods 2018, 44, 183–190. [Google Scholar] [CrossRef]
- Archaina, D.; Sosa, N.; Rivero, R.; Schebor, C. Freeze-dried candies from blackcurrant (Ribes nigrum L.) and yoghurt. Physicochemical and sensorial characterization. LWT 2019, 100, 444–449. [Google Scholar] [CrossRef]
- Díaz-García, A.; Salvá-Ruiz, B.; Bautista-Cruz, N.; Cordezo-Hoyos, L. Optimization of a natural low-calorie antioxidant tea prepared from purple corn (Zea mays L.) cobs and stevia (Stevia rebaudiana Bert.). LWT 2021, 150, 111952. [Google Scholar] [CrossRef]
- Samuel, P.; Ayoob, K.T.; Magnuson, B.A.; Wölwer-Rieck, U.; Bendix-Jeppesen, P.; Roger, P.J.; Rowland, I.; Mathews, R. Stevia leaf to stevia sweetener: Exploring its science, benefits, and future potential. J. Nutr. 2018, 148, 1186S–1205S. [Google Scholar] [CrossRef]
- Young, N.W.G.; O’Sullivan, G.R.O. The influence of ingredients on product stability and shelf life. In Food and Beverage Stability and Shelf Life; Kilcast, D., Subramaniam, P., Eds.; Woodhead Publishing: Sawston, UK, 2011; pp. 132–183. [Google Scholar]
- Grembecka, M. Sugar alcohols- their role in the modern world of sweeteners: A review. Eur. Food Res. Technol. 2015, 241, 1–14. [Google Scholar] [CrossRef]
- Joshi, K.; Kumari, A.; Arora, S.; Singh, A.K. Development of an analytical protocol for the estimation of maltitol from yoghurt, burfi and flavoured milk. LWT 2016, 70, 41–45. [Google Scholar] [CrossRef]
- Gujral, H.S.; Brar, S.S. Effect of hydrocolloids on the dehydration kinetics, color, and texture of mango leather. Int. J. Food Prop. 2007, 6, 269–279. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, A.Y.; Corfield, R.; Sosa, N.; Salvatori, D.; Schebor, C. Physicochemical, functional, and sensory characterization of apple leathers enriched with acáchul (Ardisia compressa Kunth) powder. LWT 2021, 146, 111472. [Google Scholar] [CrossRef]
- Corfield, R.; Martínez, K.D.; Allievi, M.C.; Santagapita, P.; Mazzobre, F.; Schebor, C.; Pérez, O.E. Whey protein-folic acid complexes: Formation, isolation and bioavailability in a Lactobacillus casei model. Food Struct. 2020, 26, 100162. [Google Scholar] [CrossRef]
- Corfield, R.; Lalou, G.; Di Lella, S.; Martínez, K.D.; Schebor, C.; Allievi, M.C.; Pérez, O.E. Experimental and modeling approaches applied to the whey proteins and vitamin B9 complexes study. Food Hydrocoll. 2023, 142, 108834. [Google Scholar] [CrossRef]
- Santos, D.I.; Saraiva, J.M.A.; Vicente, A.A.; Moldão-Martins, M. Methods for determining bioavailability and bioaccesibility of bioactive compounds and nutrients. In Innovative Thermal and Nonthermal Processing, Bioacessibility and Bioavailability of Nutrients and Bioactive Compounds; Barba, F., Saraiva, J.M., Cravotto, G., Lorenzo, J., Eds.; Woodhead Publishing Ltd.: Sawston, UK, 2020; pp. 23–54. [Google Scholar]
- Cilla, A.; Bosch, L.; Barberá, R.; Alegría, A. Effect of processing on the bioaccessibility of bioactive compounds—A review focusing on carotenoids, minerals, ascorbic acid, tocopherols and polyphenols. J. Food Compos. Anal. 2018, 68, 3–15. [Google Scholar] [CrossRef]
- Shahidi, F.; Peng, H. Bioaccessibility and bioavailability of phenolic compounds. J. Food Bioact. 2018, 4, 11–68. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.; Esteve, M.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Kondrashina, A.; Arranz, E.; Cilla, A.; Faria, M.; Santos-Hernández, M.; Miralles, B.; Hashemi, N.; Rasmussen, M.; Young, J.; Barberá, R.; et al. Coupling in vitro food digestion with in vitro epithelial absorption; recommendations for biocompatibility. Crit. Rev. Food Sci. Nutr. 2023, 26, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Fedi, A.; Vitale, C.; Ponschin, G.; Ayehunie, S.; Fato, M.; Scaglione, S. In vitro models replicating the human intestinal epithelium for absorption and metabolism studies: A systematic review. J. Control. Release 2021, 335, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Domínguez Rubio, A.P.; Martínez, J.; Palavecino, M.; Fuentes, F.; Sánchez López, C.M.; Marcilla, A.; Pérez, E.O.; Piuri, M. Transcytosis of Bacillus subtilis extracellular vesicles through an in vitro intestinal epithelial cell model. Sci. Rep. 2020, 10, 3120. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemist: Gaithersburg, MD, USA, 1982; Volume 1994, p. 2005. [Google Scholar]
- Código Alimentario Argentino (Argentine Food Code). Capítulo V: Normas Para la Rotulación y Publicidad de los Alimentos. 2022. Available online: https://www.argentina.gob.ar/anmat/codigoalimentario (accessed on 27 October 2023).
- Gagneten, M.; Corfield, R.; Gómez Mattson, M.; Sozzi, A.; Leiva, G.; Salvatori, D.; Schebor, C. Spray-dried powders from berries extracts obtained upon several processing steps to improve the bioactive components content. Powder Technol. 2019, 342, 1008–1015. [Google Scholar] [CrossRef]
- Sette, P.; Franceschinis, L.; Schebor, C.; Salvatori, D. Fruit snacks from raspberries: Influence of drying parameters on color degradation and bioactive potential. Int. J. Food Sci. Technol. 2017, 52, 313–328. [Google Scholar] [CrossRef]
- Koning, E.J.M. A validated liquid chromatographic method for determining folates in vegetables, milk powder, liver, and flour. J. AOAC Int. 1999, 82, 119–127. [Google Scholar] [CrossRef]
- Jiangbing, Q.; Jingrui, Z.; Aifeng, L. Cytotoxicity and intestinal permeability of phycotoxins assessed by the human Caco-2 cell model. Ecotoxicol. Environ. Saf. 2023, 249, 114447. [Google Scholar]
- Kus, M.; Ibragimow, I.; Piotrowska-Kempisty, H. Caco-2 cell line standardization with pharmaceutical requirements and in vitro model suitability for permeability assays. Pharmaceutics 2023, 15, 2523. [Google Scholar] [CrossRef]
- Torres, C.; Romero, L.; Diaz, R. Quality and sensory attributes of apple and quince leathers made without preservatives and with enhanced antioxidant activity. LWT 2015, 62, 996–1003. [Google Scholar] [CrossRef]
- KC, Y.; Dangal, A.; Thapa, S.; Rayamajhi, S.; Chalise, K.; Shiwakoti, R.; Shiwakoti, R.; Katuwal, N. Nutritional, phytochemicals, and sensory analysis of Lapsi (Choerospondias axillaris) fruit leather. Int. J. Food Prop. 2022, 25, 960–975. [Google Scholar] [CrossRef]
- Ayalew, G.M.; Emire, S.A. Formulation and characterization of fruit leather based on Annona muricata L. fruit and Avena sativa flour. J. Food Process Preserv. 2020, 44, e14284. [Google Scholar] [CrossRef]
- Ayu, D.F.; Rumengan, O.S.; Pato, U. Combination of pineapple and okra on chemical and sensory characteristics of fruit leather. IOP Conf. Ser. Earth Environ. Sci. 2020, 515, 012049. [Google Scholar] [CrossRef]
- Slavin, J.L. Dietary fiber and body weight. Nutrients 2005, 21, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T. Folate, iron, and dietary fiber contents on the gluten-free diet. J. Am. Diet. Assoc. 2000, 100, 1389–1396. [Google Scholar] [CrossRef]
- Cappa, C.; Lavelli, V.; Mariotti, M. Fruit candies enriched with grape skin powders: Physicochemical properties. LWT 2015, 62, 569–575. [Google Scholar] [CrossRef]
- Perry, J.R.; Ying, W. A review of physiological effects of soluble and insoluble dietary fibers. J. Nutr. Food Sci. 2016, 6, 476. [Google Scholar]
- Merenkova, S.P.; Zinina, O.V.; Stuart, M.; Okuskhanova, E.K.; Androsova, N.V. Effects of dietary fiber on human health: A review. Human Sport Med. 2020, 20, 106–113. [Google Scholar] [CrossRef]
- JECFA. 2019. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=267 (accessed on 20 July 2021).
- Gomez-Mattson, M.; Corfield, R.; Bajda, L.; Pérez, O.; Schebor, C.; Salvatori, D. Potential bioactive ingredient from elderberry fruit: Process optimization for a maximum phenolic recovery, physicochemical characterization, and bioaccessibility. J. Berry Res. 2021, 11, 51–68. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.L.; Páez-Hernandez, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Van Hul, M.; Cani, P.D. Targeting carbohydrates and polyphenols for a healthy microbiome and healthy weight. Curr. Nutr. Rep. 2019, 8, 307–316. [Google Scholar] [CrossRef]
- Liu, J.; He, Z.; Ma, N.; Chen, Z.Y. Beneficial effects of dietary polyphenols on high-fat diet-induced obesity linking with modulation of gut microbiota. J. Agric. Food Chem. 2019, 68, 33–47. [Google Scholar] [CrossRef]
- Wu, H.; Johnson, M.C.; Lu, C.H.; Fritsche, K.L.; Thomas, A.L.; Cai, Z.; Greenlief, C.M. Determination of anthocyanins and total polyphenols in a variety of elderberry juices by UPLC-MS/MS and other methods. Acta Hortic. 2015, 1061, 43–52. [Google Scholar] [CrossRef]
- Ochnio, M.E.; Martínez, J.H.; Allievi, M.C.; Palavecino, M.; Martínez, K.D.; Pérez, O.E. Proteins as nano-carriers for bioactive compounds. The case of 7S and 11S soy globulins and folic acid complexation. Polymers 2018, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.B.; Zevely, E.M.; Huennekens, F.M. Coupling of energy to folate transport in Lactobacillus casei. J. Bacteriol. 1979, 139, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Eudes, A.; Erkens, G.B.; Slotboom, D.J.; Rodionov, D.A.; Naponelli, V.; Hanson, A.D. Identification of genes encoding the folate and thiamine binding membrane proteins in firmicutes. J. Bacteriol. 2008, 190, 7591–7759. [Google Scholar] [CrossRef]
- Kunji, E.R.S.; Mierau, I.; Hagting, A.; Poolman, B.; Konings, W.N. The proteolytic system of lactic acid bacteria. Antonie Van Leeuwenhoek 1996, 70, 187–221. [Google Scholar] [CrossRef] [PubMed]
- Alcántara, C.; Bäuerl, C.; Revilla-Guarinos, A.; Pérez-Martínez, G.; Monedero, V.; Zúñiga, M. Peptide and amino acid metabolism is controlled by an OmpR-family response regulator in Lactobacillus casei. Mol. Microbiol. 2016, 100, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Aceituno-Medina, M.; Mendoza, S.; Lagaron, J.M.; López-Rubio, A. Photoprotection of folic acid upon encapsulation in food-grade amaranth (Amaranthus hypochondriacus L.) protein isolate-pullulan electrospun fibers. LWT 2015, 62, 970–975. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Crizel, R.L.; da Silva, F.T.; Fontes, M.R.V.; Zavareze, E.; Días, A.R.G. Starch nanofibers as vehicles for folic acid supplementation: Thermal treatment, UVA irradiation and in vitro simulation of digestion. J. Sci. Food Agric. 2020, 101, 1935–1943. [Google Scholar] [CrossRef]
- Koning, E.J.M.; Roomans, H.H.S.; Dorant, E.; Goldbohm, R.A.; Saris, W.H.M.; van den Brandt, P.A. Folate intake of the Dutch population according to newly established liquid chromatography data for foods. Am. J. Clin. Nutr. 2001, 73, 765–776. [Google Scholar] [CrossRef]
- Kariluoto, S.; Vahteristo, L.; Salovaara, H.; Katina, K.; Liukkonen, K.H.; Piironen, V. Effect of baking method and fermentation on folate content of rye and wheat wreads. Cereal Chem. 2004, 81, 134–139. [Google Scholar] [CrossRef]
- Olivares, A.B.; Bernal, M.J.; Ros, G.; Martínez, C.; Periago, M.J. Folatos y su disponibilidad en alimentos evaluados por cromatografía líquida (HPLC). Validación y aplicaciones en calidad nutricional de alimentos. An. Vet. Murcia 2004, 20, 59–73. [Google Scholar]
- Delchier, N.; Ringling, C.; Maingonnat, J.F.; Rychlik, M.; Renard, C. Mechanisms of folate losses during processing: Diffusion vs. heat degradation. Food Chem. 2014, 157, 439–447. [Google Scholar] [CrossRef]
- Liu, F.; Kariluoto, S.; Edelmann, M.; Piironen, V. Bioaccessibility of folate in faba bean, oat, rye and wheat matrices. Food Chem. 2021, 350, 129259. [Google Scholar] [CrossRef] [PubMed]
- Stellaard, F.; Lütjohann, D. Dynamics of the enterohepatic circulation of bile acids in healthy humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Miao, T.; Song, G.; Yang, J. Protective effect of apple polyphenols on H2O2-induced oxidative stress damage in human colon adenocarcinoma Caco-2 cells. Chem. Pharm. Bull. 2023, 71, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Bellion, P.; Digles, J.; Will, F.; Dietrich, H.; Baum, M.; Eisenbrand, G.; Janzowski, C. Polyphenolic apple extracts: Effects of raw material and production method on antioxidant effectiveness and reduction of DNA damage in Caco-2 cells. J. Agric. Food Chem. 2010, 58, 6636–6642. [Google Scholar] [CrossRef]
- Ferreira, S.; Martins-Gomes, C.; Nunes, F.; Silva, A. Elderberry (Sambucus nigra L.) extracts promote anti-inflammatory and cellular antioxidant activity. Food Chem. X 2022, 15, 100437. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Y.; Zhang, L.; Li, Y.; Zheng, X. Wild raspberry subjected to simulated gastrointestinal digestion improves the protective capacity against ethyl carbamate-induced oxidative damage in Caco-2 cells. Oxid. Med. Cell. Longev. 2016, 2016, 3297363. [Google Scholar] [CrossRef]



| Components | (g/100 g) |
|---|---|
| Apple puree | 90.16 |
| Acáchul powder | 1.0 |
| Maltitol syrup | 8.5 |
| Stevia | 0.33 |
| WPI-FA | 0.0074 |
| Composition | Values (g/100 g) |
|---|---|
| Carbohydrates | 70.03 ± 4.6 |
| Crude Fiber | 5.29 ± 0.69 |
| Protein | 0.88 ± 0.20 |
| Fat | 0.12 ± 0.03 |
| Ash | 4.70 ± 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corfield, R.; Allievi, M.C.; Rivero, R.; López, T.A.; Pérez, O.E.; Salvatori, D.; Schebor, C. An Apple and Acáchul Berry Snack Rich in Bioaccessible Antioxidants and Folic Acid: A Healthy Alternative for Prenatal Diets. Foods 2024, 13, 692. https://doi.org/10.3390/foods13050692
Corfield R, Allievi MC, Rivero R, López TA, Pérez OE, Salvatori D, Schebor C. An Apple and Acáchul Berry Snack Rich in Bioaccessible Antioxidants and Folic Acid: A Healthy Alternative for Prenatal Diets. Foods. 2024; 13(5):692. https://doi.org/10.3390/foods13050692
Chicago/Turabian StyleCorfield, Rocío, Mariana C. Allievi, Roy Rivero, Tamara A. López, Oscar E. Pérez, Daniela Salvatori, and Carolina Schebor. 2024. "An Apple and Acáchul Berry Snack Rich in Bioaccessible Antioxidants and Folic Acid: A Healthy Alternative for Prenatal Diets" Foods 13, no. 5: 692. https://doi.org/10.3390/foods13050692