Encapsulation of Benzaldehyde Produced by the Eco-Friendly Degradation of Amygdalin in the Apricot Kernel Debitterizing Wastewater
Abstract
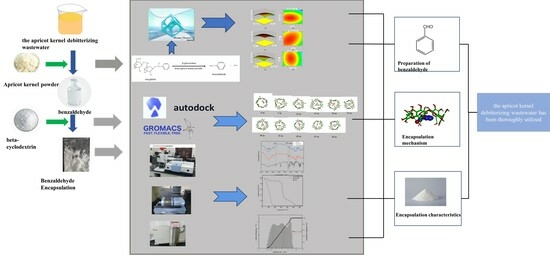
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Benzaldehyde
2.3. Determination of Benzaldehyde Amygdalin and Hydrocyanic Acid Content
2.4. Process Optimization of Degrading Amygdalin into Benzaldehyde by Response Surface Methodology
2.5. Preparation of the bz-β-CD
2.6. Characterization of the bz-β-CD Encapsulation Effect
2.6.1. Determination of Encapsulation Rate
2.6.2. The Effects of Different Wall–Core Ratios on Encapsulation Rate
2.6.3. Fourier Transform Infrared Spectroscopy Analysis (FI-IR)
2.6.4. Thermo-Gravimetric Analysis (TGA)
2.6.5. Particle Size Distribution Analysis
2.6.6. Release Kinetics Analysis
2.6.7. Inhibition Experiment of Mycelial Growth of Botrytis cinerea
2.7. Molecular Simulation
2.7.1. Molecular Docking Simulation
2.7.2. Molecular Dynamics Simulation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Benzaldehyde Preparation Process
3.2. Characterization of Benzaldehyde Encapsulation by β-CD
3.2.1. The Effects of Different Wall–Core Ratios on the Encapsulation Rate of bz-β-CD
3.2.2. Fourier Transform Infrared Spectroscopy Analysis of bz-β-CD
3.2.3. Thermogravimetric Analysis of β-CD, Benzaldehyde, and bz-β-CD
3.2.4. Particle Size Analysis of bz-β-CD
3.2.5. Effects of Different Temperatures on the Release Kinetics of bz-β-CD
3.2.6. Effects of Different Concentrations of bz and bz-β-CD on Antifungal Activity of Botrytis cinerea
3.3. The Analysis of the Encapsulation Mechanism of Benzaldehyde and β-CD
3.3.1. Analysis of Molecular Docking Results of Benzaldehyde and β-CD
3.3.2. The Impacts of Temporal Variations on Conformation
3.3.3. Root Mean Square Deviation Analysis
3.3.4. Radius of Gyration Analysis of bz-β-CD
3.3.5. Interaction Energy Analysis of bz-β-CD
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, T.; Lu, S.; Xia, Q.; Fang, Z.; Johnson, S. Separation and purification of amygdalin from thinned bayberry kernels by macroporous adsorption resins. J. Chromatogr. B 2015, 975, 52–58. [Google Scholar] [CrossRef]
- Zhang, Q.-A.; Song, Y.; Wang, X.; Zhao, W.-Q.; Fan, X.-H. Mathematical modeling of debittered apricot (Prunus armeniaca L.) kernels during thin-layer drying. CyTA-J. Food 2016, 14, 509–517. [Google Scholar] [CrossRef]
- Zhang, Q.; Yao, L. State-of-the-art on the processing and comprehensive utilization of the apricot kernels. Sci. Agric. Sin 2019, 52, 3430–3447. [Google Scholar]
- Zhang, Q.-A.; Wei, C.-X.; Fan, X.-H.; Shi, F.-F. Chemical compositions and antioxidant capacity of by-products generated during the apricot kernels processing. CyTA-J. Food 2018, 16, 422–428. [Google Scholar] [CrossRef]
- Fan, X.-H.; Zhang, X.-Y.; Zhang, Q.-A.; Zhao, W.-Q.; Shi, F.-F. Optimization of ultrasound parameters and its effect on the properties of the activity of beta-glucosidase in apricot kernels. Ultrason. Sonochem. 2019, 52, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Calvo, H.; Mendiara, I.; Arias, E.; Gracia, A.P.; Blanco, D.; Venturini, M.E. Antifungal activity of the volatile organic compounds produced by Bacillus velezensis strains against postharvest fungal pathogens. Postharvest Biol. Technol. 2020, 166, 111208. [Google Scholar] [CrossRef]
- Lee, H.h.; Ahn, J.H.; Kwon, A.R.; Lee, E.S.; Kwak, J.H.; Min, Y.H. Chemical composition and antimicrobial activity of the essential oil of apricot seed. Phytother. Res. 2014, 28, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Khan, A.L.; Ali, L.; Khan, A.R.; Waqas, M.; Hussain, J.; Lee, I.-J.; Shin, J.-H. Benzaldehyde as an insecticidal, antimicrobial, and antioxidant compound produced by Photorhabdus temperata M1021. J. Microbiol. 2015, 53, 127–133. [Google Scholar] [CrossRef]
- Yang, Z.-J.; Jiang, H.-G.; Zhou, X.-T.; Fang, Y.-X.; Ji, H.-B. β-Cyclodextrin polymer promoted green synthesis of cinnamaldehyde to natural benzaldehyde in aqueous solution. Supramol. Chem. 2012, 24, 379–384. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, D.; Ge, S.; Bian, P.; Xue, H.; Lang, Y. Alginate-based edible coating with oregano essential oil/β-cyclodextrin inclusion complex for chicken breast preservation. Int. J. Biol. Macromol. 2023, 251, 126126. [Google Scholar] [CrossRef]
- Zhang, W.; Ezati, P.; Khan, A.; Assadpour, E.; Rhim, J.-W.; Jafari, S.M. Encapsulation and delivery systems of cinnamon essential oil for food preservation applications. Adv. Colloid Interface Sci. 2023, 318, 102965. [Google Scholar] [CrossRef]
- Szejtli, J. Past, present and futute of cyclodextrin research. Pure Appl. Chem. 2004, 76, 1825–1845. [Google Scholar] [CrossRef]
- Rukmani, A.; Sundrarajan, M. Inclusion of antibacterial agent thymol on β-cyclodextrin-grafted organic cotton. J. Ind. Text. 2012, 42, 132–144. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Q.-A.; Yao, J.-L. Consecutive membrane filtration and re-utilization of the debitterizing wastewater of apricot kernels for a flavor beverage-making. J. Clean. Prod. 2020, 262, 121360. [Google Scholar] [CrossRef]
- Zhang, Q.-A.; Shi, F.-F.; Yao, J.-L.; Zhang, N. Effects of ultrasound irradiation on the properties of apricot kernels during accelerated debitterizing. RSC Adv. 2020, 10, 10624–10633. [Google Scholar] [CrossRef]
- Nakamura, E.; Yagi, M. Spectrophotometric Determination of Cyanide with Isonicotinic Acid-Sodium Barbiturate. Bunseki Kagaku 2000, 49, 55–58. [Google Scholar] [CrossRef]
- Abarca, R.L.; Rodriguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of beta-cyclodextrin inclusion complexes containing an essential oil component. Food Chem. 2016, 196, 968–975. [Google Scholar] [CrossRef]
- Heydari, M.K.; Assadpour, E.; Jafari, S.M.; Javadian, H. Encapsulation of rose essential oil using whey protein concentrate-pectin nanocomplexes: Optimization of the effective parameters. Food Chem. 2021, 356, 129731. [Google Scholar] [CrossRef]
- Ren, X.; Yue, S.; Xiang, H.; Xie, M. Inclusion complexes of eucalyptus essential oil with β-cyclodextrin: Preparation, characterization and controlled release. J. Porous Mater. 2018, 25, 1577–1586. [Google Scholar] [CrossRef]
- Edwards, S.G.; Seddon, B. Mode of antagonism of Brevibacillus brevis against Botrytis cinerea in vitro. J. Appl. Microbiol. 2010, 91, 652–659. [Google Scholar] [CrossRef]
- Muhammad, E.F.; Adnan, R.; Latif, M.A.M.; Abdul Rahman, M.B. Theoretical investigation on insulin dimer-β-cyclodextrin interactions using docking and molecular dynamics simulation. J. Incl. Phenom. Macrocycl. Chem. 2016, 84, 1–10. [Google Scholar] [CrossRef]
- Fateminasab, F.; Bordbar, A.; Shityakov, S.; Saboury, A. Molecular insights into inclusion complex formation between β-and γ-cyclodextrins and rosmarinic acid. J. Mol. Liq. 2020, 314, 113802. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Liu, Q. FT-IR spectroscopy and DFT calculation study on the solvent effects of benzaldehyde in organic solvents. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 86, 51–55. [Google Scholar] [CrossRef]
- Qiao, X.; Yang, L.; Hu, X.; Cao, Y.; Xue, C. Characterization and evaluation of inclusion complexes between astaxanthin esters with different molecular structures and hydroxypropyl-β-cyclodextrin. Food Hydrocoll. 2020, 110, 106208. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrohydrodynamic encapsulation of eugenol-cyclodextrin complexes in pullulan nanofibers. Food Hydrocoll. 2020, 111, 106264. [Google Scholar] [CrossRef]
- Lu, H.; Wang, Y.; Xie, X.; Chen, F.; Li, W. Molecular Dynamics Simulation and TDDFT Study of the Structures and Uv-vis Absorption Spectra of MCT-β-CD and its Inclusion Complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Ohga, K.; Takashima, Y.; Takahashi, H.; Kawaguchi, Y.; Yamaguchi, H.; Harada, A. Preparation of Supramolecular Polymers from a Cyclodextrin Dimer and Ditopic Guest Molecules: Control of Structure by Linker Flexibility. Macromolecules 2005, 38, 5897–5904. [Google Scholar] [CrossRef]
- Sangpheak, W.; Khuntawee, W.; Wolschann, P.; Pongsawasdi, P.; Rungrotmongkol, T. Enhanced stability of a naringenin/2,6-dimethyl β-cyclodextrin inclusion complex: Molecular dynamics and free energy calculations based on MM-and QM-PBSA/GBSA. J. Mol. Graph. Model. 2014, 50, 10–15. [Google Scholar] [CrossRef] [PubMed]







| Independent Variables | Factor | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1—Time (h) | 1.5 | 2 | 2.5 |
| X2—Temperature (°C) | 65 | 55 | 45 |
| X3—pH | 5 | 6 | 7 |
| Test | X1—Time (h) | X2—Temperature (°C) | X3—pH | Y—Benzaldehyde (g/L) |
|---|---|---|---|---|
| 1 | 2 | 45 | 5 | 1.674874309 |
| 2 | 1.5 | 45 | 6 | 1.726983809 |
| 3 | 2.5 | 45 | 6 | 1.662509763 |
| 4 | 2 | 45 | 7 | 1.740413837 |
| 5 | 2.5 | 55 | 5 | 1.496666262 |
| 6 | 1.5 | 55 | 5 | 2.11229181 |
| 7 | 2 | 55 | 6 | 2.169431375 |
| 8 | 2 | 55 | 6 | 2.14237309 |
| 9 | 2 | 55 | 6 | 2.053541632 |
| 10 | 2.5 | 55 | 7 | 1.757337655 |
| 11 | 1.5 | 55 | 7 | 1.848597421 |
| 12 | 2 | 65 | 5 | 1.469880542 |
| 13 | 2.5 | 65 | 6 | 1.302500764 |
| 14 | 1.5 | 65 | 6 | 1.397502106 |
| 15 | 2 | 65 | 7 | 1.514259103 |
| Source | SS | df | MS | F Value | p Value | |
|---|---|---|---|---|---|---|
| Model | 1.11 | 9 | 0.12 | 13.95 | 0.0049 | ** |
| X1 | 0.099 | 1 | 0.099 | 11.27 | 0.0202 | * |
| X2 | 0.18 | 1 | 0.18 | 20.02 | 0.0066 | ** |
| X3 | 2.781 × 10−5 | 1 | 2.781 × 10−5 | 3.157 × 10−3 | 0.9574 | |
| X1X2 | 7.647 × 10−4 | 1 | 7.647 × 10−4 | 0.087 | 0.7801 | |
| X1X3 | 0.069 | 1 | 0.069 | 7.80 | 0.0383 | * |
| X2X3 | 6.618 × 10−3 | 1 | 6.618 × 10−3 | 0.75 | 0.4258 | |
| X12 | 0.12 | 1 | 0.12 | 13.13 | 0.0152 | |
| X22 | 0.64 | 1 | 0.64 | 72.60 | 0.0004 | |
| X32 | 0.073 | 1 | 0.073 | 8.34 | 0.0343 | |
| Residual | 0.044 | 5 | 8.811 × 10−3 | |||
| Lack of Fit | 0.037 | 3 | 0.012 | 3.33 | 0.2395 | |
| Pure Error | 7.351 × 10−3 | 2 | 3.676 × 10−3 | |||
| Cor Total | 1.15 | 14 |
| Temperature (°C) | Fitting Equation | R2 | |
|---|---|---|---|
| Avrami’s fitting | 5 | y = 0.54665x − 1.88522 | 0.91091 |
| 30 | y = 0.59824x − 1.80598 | 0.90536 | |
| 55 | y = 0.62519x − 1.73751 | 0.95358 | |
| Zero-order fitting | 5 | y = 3.98832x + 6.76983 | 0.91297 |
| 30 | y = 4.65976x + 7.24012 | 0.92636 | |
| 55 | y = 5.26538x + 7.75528 | 0.93743 | |
| First-order fitting | 5 | y = −0.5382x − 0.0598 | 0.94713 |
| 30 | y = −0.6676x − 0.05795 | 0.96374 | |
| 55 | y = −0.7991x − 0.05568 | 0.97764 |
| Energy | Complex |
|---|---|
| Van der Waals energy (KJ/mol) | −77.278 |
| Electrostatic energy (KJ/mol) | −13.282 |
| Polar solvation energy (KJ/mol) | 39.041 |
| Nonpolar solvation Energy (KJ/mol) | −9.829 |
| Total binding energy (KJ/mol) | −61.348 |
| T∆S (KJ/mol) | 9.615 |
| Total binding free energy (KJ/mol) | −51.733 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; García Martín, J.F.; Zhang, Q.-A. Encapsulation of Benzaldehyde Produced by the Eco-Friendly Degradation of Amygdalin in the Apricot Kernel Debitterizing Wastewater. Foods 2024, 13, 437. https://doi.org/10.3390/foods13030437
Song L, García Martín JF, Zhang Q-A. Encapsulation of Benzaldehyde Produced by the Eco-Friendly Degradation of Amygdalin in the Apricot Kernel Debitterizing Wastewater. Foods. 2024; 13(3):437. https://doi.org/10.3390/foods13030437
Chicago/Turabian StyleSong, Lei, Juan Francisco García Martín, and Qing-An Zhang. 2024. "Encapsulation of Benzaldehyde Produced by the Eco-Friendly Degradation of Amygdalin in the Apricot Kernel Debitterizing Wastewater" Foods 13, no. 3: 437. https://doi.org/10.3390/foods13030437