Facile Preparation of Magnetic COF-on-COF for Rapid Adsorption and Determination of Sulforaphane from Cruciferous Vegetables
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Instruments and HPLC-MS/MS Conditions
2.3. Preparation of Standard Solutions
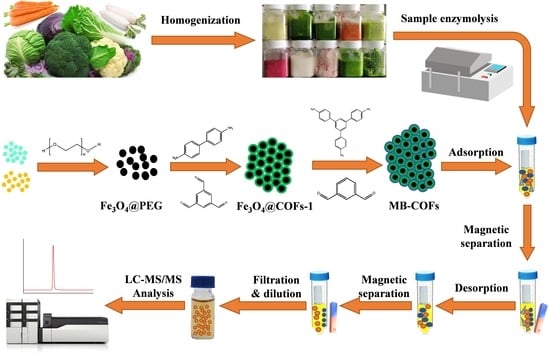
2.4. Preparation of MB-COFs
2.4.1. Preparation of Fe3O4@PEG
2.4.2. Preparation of Fe3O4@COFs-1
2.4.3. Preparation of Fe3O4@COF-on-COF
2.5. Sample Preparation
2.5.1. Sample Enzymolysis
2.5.2. MSPE Procedure
2.6. Adsorption Experiment
2.6.1. Optimization of the MSPE Conditions
2.6.2. Adsorption Kinetics
2.6.3. Adsorption Isotherm
2.7. Method Validation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Preparation, Optimization, and Characterization of MB-COFs
3.1.1. SEM and TEM Analysis
3.1.2. XRD Analysis
3.1.3. FT-IR Analysis
3.1.4. VSM Analysis
3.1.5. N2 Adsorption–Desorption Isotherms
3.1.6. XPS Analysis
3.2. Optimization of MSPE Procedures
3.2.1. Adsorbent Amount
3.2.2. Adsorption Time
3.2.3. pH
3.2.4. Ionic Strength
3.2.5. Temperature
3.2.6. Desorption Solvent
3.2.7. Desorption Time
3.2.8. Desorption Solvent Volume
3.2.9. Reusability
3.3. Adsorption Kinetics
3.4. Adsorption Isotherm
3.5. Adsorption Thermodynamics
3.6. Adsorption Mechanism
3.7. Method Validation and Comparison
3.8. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sikorska-Zimny, K.; Beneduce, L. The glucosinolates and their bioactive derivatives in Brassica: A review on classification, biosynthesis and content in plant tissues, fate during and after processing, effect on the human organism and interaction with the gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 2544–2571. [Google Scholar] [CrossRef]
- Royce, S.G.; Licciardi, P.V.; Beh, R.C.; Bourke, J.E.; Donovan, C.; Hung, A.N.; Khurana, I.; Liang, J.L.J.; Maxwell, S.; Mazarakis, N.; et al. Sulforaphane prevents and reverses allergic airways disease in mice via anti-inflammatory, antioxidant, and epigenetic mechanisms. Cell. Mol. Life Sci. 2022, 79, 1–22. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Zhang, N.; Li, Y.; Cai, Z.; Li, G.; Liu, Z.; Liu, Z.; Wang, Y.; Shao, X.; et al. Anti-aging activity and their mechanisms of natural food-derived peptides: Current advancements. Food Innov. Adv. 2023, 2, 272–290. [Google Scholar] [CrossRef]
- Fusari, C.M.; Ramirez, D.A.; Camargo, A.B. Simplified analytical methodology for glucosinolate hydrolysis products: A miniaturized extraction technique and multivariate optimization. Anal. Methods 2019, 11, 309–316. [Google Scholar] [CrossRef]
- Franco, P.; Spinozzi, S.; Pagnotta, E.; Lazzeri, L.; Ugolini, L.; Camborata, C.; Roda, A. Development of a liquid chromatography-electrospray ionization-tandem mass spectrometry method for the simultaneous analysis of intact glucosinolates and isothiocyanates in Brassicaceae seeds and functional foods. J. Chromatogr. A 2016, 1428, 154–161. [Google Scholar] [CrossRef]
- Chiang, W.C.K.; Pusateri, D.J.; Leitz, R.E.A. Gas chromatography mass spectrometry method for the determination of sulforaphane and sulforaphane nitrile in broccoli. J. Agric. Food Chem. 1998, 46, 1018–1021. [Google Scholar] [CrossRef]
- ISO 9167:2019; Rapeseed and Rapeseed Meals—Determination of Glucosinolates Content—Method Using High-Performance Liquid Chromatography. International Organization for Standardization (ISO): Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/72207.html (accessed on 25 January 2024).
- Ares, A.M.; Valverde, S.; Bernal, J.L.; Nozal, M.J.; Bernal, J. Development and validation of a LC-MS/MS method to determine sulforaphane in honey. Food Chem. 2015, 181, 263–269. [Google Scholar] [CrossRef]
- NY/T 3674-2020; Determination of Sulforphane in Brassica Napus-Liquid Chromatography-Tandem Mass Spectrometry. Ministry of Agriculture and Rural Affairs (MARA) of the People’s Republic of China: Beijing, China, 2020.
- Liu, Y.; Zhang, D.; Li, X.; Xiao, J.; Guo, L. Enhancement of ultrasound-assisted extraction of sulforaphane from broccoli seeds via the application of microwave pretreatment. Ultrason. Sonochem. 2022, 87, 106061. [Google Scholar] [CrossRef]
- Saylan, M.; Demirel, R.; Ayyildiz, M.F.; Chormey, D.S.; Cetin, G.; Bakirdere, S. Nickel hydroxide nanoflower-based dispersive solid-phase extraction of copper from water matrix. Environ. Monit. Assess. 2022, 195, 133. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Pasandi, S.; Mohebbi, A.; Afshar Mogaddam, M.R. Application of magnetic iron (III) oxinate nanocomposite as an efficient sorbent in magnetic dispersive solid phase extraction of pesticides. Microchem. J. 2022, 179, 107584. [Google Scholar] [CrossRef]
- Liu, X.L.; Li, Y.; Chen, Z.S.; Yang, H.; Cai, Y.W.; Wang, S.H.; Chen, J.R.; Hu, B.W.; Huang, Q.F.; Shen, C.; et al. Advanced porous nanomaterials as superior adsorbents for environmental pollutants removal from aqueous solutions. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1289–1309. [Google Scholar] [CrossRef]
- Xiao, Y.; Ahmad, T.; Belwal, T.; Aadil, R.M.; Siddique, M.; Pang, L.; Xu, Y. A review on protein based nanocarriers for polyphenols: Interaction and stabilization mechanisms. Food Innov. Adv. 2023, 2, 193–202. [Google Scholar] [CrossRef]
- Javed, M.; Matloob, A.; Ettoumi, F.-e.; Sheikh, A.R.; Zhang, R.; Xu, Y. Novel nanobubble technology in food science: Application and mechanism. Food Innov. Adv. 2023, 2, 135–144. [Google Scholar] [CrossRef]
- He, J.; Luo, B.; Zhang, H.; Li, Z.; Zhu, N.; Lan, F.; Wu, Y. Surfactant-free synthesis of covalent organic framework nanospheres in water at room temperature. J. Colloid Interface Sci. 2022, 606, 1333–1339. [Google Scholar] [CrossRef]
- Gao, M.; Deng, L.; Kang, X.; Fu, Q.; Zhang, K.; Wang, M.; Xia, Z.; Gao, D. Core-shell structured magnetic covalent organic frameworks for magnetic solid-phase extraction of diphenylamine and its analogs. J. Chromatogr. A 2020, 1629, 461476. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; He, J.; Yang, R.; Ma, X.; Yuan, Y.; Yue, T.; Sheng, Q. Functionalized magnetic covalent organic framework nanocomposites for high-efficiency adsorption of ethyl carbamate from liquor. Food Front. 2023, 4, 911–921. [Google Scholar] [CrossRef]
- Wang, J.X.; Li, J.; Gao, M.X.; Zhang, X.M. Recent advances in covalent organic frameworks for separation and analysis of complex samples. Trac -Trends Anal. Chem. 2018, 108, 98–109. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, L.; Liu, G.; Liu, Y.; Zhang, S.; Wang, L.; Zheng, X.; Zhou, L.; Gao, J.; Shi, J.; et al. Hollow Rh-COF@COF S-Scheme Heterojunction for Photocatalytic Nicotinamide Cofactor Regeneration. ACS Catal. 2023, 13, 6619–6629. [Google Scholar] [CrossRef]
- Fan, H.; Mundstock, A.; Feldhoff, A.; Knebel, A.; Gu, J.; Meng, H.; Caro, J. Covalent Organic Framework-Covalent Organic Framework Bilayer Membranes for Highly Selective Gas Separation. J. Am. Chem. Soc. 2018, 140, 10094–10098. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, M.; Chen, G.; Tian, M.; Zhai, R.; Huang, X.; Xu, X.; Liu, G.; Xu, D. Facile synthesis of covalent organic frameworks functionalized with graphene hydrogel for effectively extracting organophosphorus pesticides from vegetables. Food Chem. 2021, 352, 129187. [Google Scholar] [CrossRef]
- Yan, Y.; Lu, Y.; Wang, B.; Gao, Y.; Zhao, L.; Liang, H.; Wu, D. Self-Assembling Hydrophilic Magnetic Covalent Organic Framework Nanospheres as a Novel Matrix for Phthalate Ester Recognition. ACS Appl. Mater. Interfaces 2018, 10, 26539–26545. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, C.; Tan, Q.; Gao, M.; Chen, G.; Zhai, R.; Huang, X.; Xu, X.; Liu, G.; Wang, J.; et al. Ternary magnetic Fe3O4@C3N4@covalent organic framework for facile extraction and determination of organophosphorus pesticides in fruit. Microchem. J. 2022, 174, 107103. [Google Scholar] [CrossRef]
- Kokotou, M.G.; Revelou, P.K.; Pappas, C.; Constantinou-Kokotou, V. High resolution mass spectrometry studies of sulforaphane and indole-3-carbinol in broccoli. Food Chem. 2017, 237, 566–573. [Google Scholar] [CrossRef]
- Chen, R.; Qiao, X.; Liu, F.; Chen, X. Amino acid ionic liquid-based magnetic dispersive solid-phase extraction for benzimidazole residue analysis in fruit juice and human serum based on theoretical screening. Food Chem. 2023, 404, 134695. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA), USA. Guidance for Industry: Q2(R1) Validation of Analytical Procedures: Text and Methodology. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q2r1-validation-analytical-procedures-text-and-methodology-guidance-industry (accessed on 25 January 2024).
- Fang, L.; Qiu, F. Determination of neurotoxic shellfish poisoning toxins in shellfish by liquid chromatography-tandem mass spectrometry coupled with dispersive solid phase extraction. Heliyon 2023, 9, e21610. [Google Scholar] [CrossRef]
- Wang, M.; Gao, M.; Zhang, K.; Wang, L.; Wang, W.; Fu, Q.; Xia, Z.; Gao, D. Magnetic covalent organic frameworks with core-shell structure as sorbents for solid phase extraction of fluoroquinolones, and their quantitation by HPLC. Mikrochim. Acta 2019, 186, 827. [Google Scholar] [CrossRef]
- Shkinev, V.; Maksimova, V.; Mokhodoeva, O.; Larichev, V.; Spivakov, B.; Osmolovskaya, O.; Egorova, A.; Smirnova, I.; Dzhenloda, R. Nanosized magnetite modified with poly(ethylene glycol) for efficient sorption of L-lysine-α-oxidase from the culture fluid. Mater. Lett. 2022, 323, 132535. [Google Scholar] [CrossRef]
- Liu, G.; Huang, X.; Lu, M.; Li, L.; Li, T.; Xu, D. Facile synthesis of magnetic zinc metal-organic framework for extraction of nitrogen-containing heterocyclic fungicides from lettuce vegetable samples. J. Sep. Sci. 2019, 42, 1451–1458. [Google Scholar] [CrossRef]
- Albalawi, A.E.; Khalaf, A.K.; Alyousif, M.S.; Alanazi, A.D.; Baharvand, P.; Shakibaie, M.; Mahmoudvand, H. Fe3O4(@)piroctone olamine magnetic nanoparticles: Synthesize and therapeutic potential in cutaneous leishmaniasis. Biomed. Pharmacother. 2021, 139, 111566. [Google Scholar] [CrossRef]
- Guo, H.; Li, Y.; Li, Y.; He, X.; Chen, L.; Zhang, Y. Construction of Stable Magnetic Vinylene-Linked Covalent Organic Frameworks for Efficient Extraction of Benzimidazole Fungicides. ACS Appl. Mater. Interfaces 2023, 15, 14777–14787. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.Q.; Ge, P.; Zhang, B.; Wen, L.; Gu, C.H.; Zhou, X. Sulforaphene: Formation, Stability, Separation, Purification, Determination and Biological Activities. Sep. Purif. Rev. 2022, 51, 330–339. [Google Scholar] [CrossRef]
- Wu, Y.; Mao, J.; You, Y.; Liu, S. Study on degradation kinetics of sulforaphane in broccoli extract. Food Chem. 2014, 155, 235–239. [Google Scholar] [CrossRef]
- Tan, C.; Li, J.; Liu, W.; Zhao, Q.; Wang, X.; Li, Y. Core-shell magnetic covalent organic framework nanocomposites as an adsorbent for effervescent reaction-enhanced microextraction of endocrine disruptors in liquid matrices. Chem. Eng. J. 2020, 396, 125191. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhang, H.N.; Chen, Y.T.; Huang, L.; Lin, Z.; Cai, Z.W. Core-Shell Structured Magnetic Covalent Organic Framework Nanocomposites for Triclosan and Triclocarban Adsorption. ACS Appl. Mater. Interfaces 2019, 11, 22492–22500. [Google Scholar] [CrossRef]
- Almeida, A.C.M.; do Nascimento, R.A.; Amador, I.C.B.; Santos, T.C.d.S.; Martelli, M.C.; de Faria, L.J.G.; Ribeiro, N.F.d.P. Chemically activated red mud: Assessing structural modifications and optimizing adsorption properties for hexavalent chromium. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628. [Google Scholar] [CrossRef]
- Schwaab, M.; Steffani, E.; Barbosa-Coutinho, E.; Severo, J.B. Critical analysis of adsorption/diffusion modelling as a function of time square root. Chem. Eng. Sci. 2017, 173, 179–186. [Google Scholar] [CrossRef]
- Ozer, A.; Akkaya, G.; Turabik, M. The biosorption of Acid Red 337 and Acid Blue 324 on Enteromorpha prolifera: The application of nonlinear regression analysis to dye biosorption. Chem. Eng. J. 2005, 112, 181–190. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, Y.H.; Lv, F.Z.; Chu, P.K.; Shang, J.W. Removal of organic materials from TNT red water by Bamboo Charcoal adsorption. Chem. Eng. J. 2012, 193, 39–49. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Y.; Shi, Z.; Sun, X.; Bu, T.; Zhang, C.; Mao, Z.; Li, X.; Wang, L. Magnetic amino-functionalized-MOF(M = Fe, Ti, Zr)@COFs with superior biocompatibility: Performance and mechanism on adsorption of azo dyes in soft drinks. Chem. Eng. J. 2021, 420. [Google Scholar] [CrossRef]
- Qin, P.; Chen, D.; Li, D.; Li, M.; Mu, M.; Gao, Y.; Zhu, S.; Lu, M. Synthesis of spindle-like amino-modified Zn/Fe bimetallic metal-organic frameworks as sorbents for dispersive solid-phase extraction and preconcentration of phytohormoes in vegetable samples. Food Chem 2023, 409, 135272. [Google Scholar] [CrossRef]
- Lima, J.Z.; Ferreira da Silva, E.; Patinha, C.; Duraes, N.; Vieira, E.M.; Rodrigues, V.G.S. Sorption of arsenic by composts and biochars derived from the organic fraction of municipal solid wastes: Kinetic, isotherm and oral bioaccessibility study. Environ. Res. 2022, 204, 111988. [Google Scholar] [CrossRef]
- Zou, C.; Xu, Z.; Nie, F.; Guan, K.; Li, J. Application of hydroxyapatite-modified carbonized rice husk for the adsorption of Cr(VI) from aqueous solution. J. Mol. Liq. 2023, 371, 121137. [Google Scholar] [CrossRef]
- De Oliveira, C.; Renda, C.G.; Moreira, A.J.; Pereira, O.A.P.; Pereira, E.C.; Freschi, G.P.G.; Bertholdo, R. Evaluation of a graphitic porous carbon modified with iron oxides for atrazine environmental remediation in water by adsorption. Environ. Res. 2023, 219, 115054. [Google Scholar] [CrossRef]
- Barkakati, P.; Begum, A.; Das, M.L.; Rao, P.G. Adsorptive separation of Ginsenoside from aqueous solution by polymeric resins: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2010, 161, 34–45. [Google Scholar] [CrossRef]
- Hassan, R.; Abo Eldahab, H.M.M.; Shehata, F.A.; El-Reefy, S.A.; Mohamed, S.A. Proficiency of some synthetic alginate derivatives for sequestration of Iodine-131 from radioactive liquid waste. Environ. Technol. 2023, 1–14. [Google Scholar] [CrossRef]
- Zhao, J.; Dai, Y. Tetracycline adsorption mechanisms by NaOH-modified biochar derived from waste Auricularia auricula dregs. Environ. Sci. Pollut. Res. Int. 2022, 29, 9142–9152. [Google Scholar] [CrossRef]
- ZA, A.L.; Badjah, A.Y.; Alharbi, O.M.L.; Ali, I. Copper carboxymethyl cellulose nanoparticles for efficient removal of tetracycline antibiotics in water. Environ. Sci. Pollut. Res. Int. 2020, 27, 42960–42968. [Google Scholar] [CrossRef]
- Baggiani, C.; Giraudi, G.; Giovannoli, C.; Tozzi, C.; Anfossi, L. Adsorption isotherms of a molecular imprinted polymer prepared in the presence of a polymerisable template. Anal. Chim. Acta 2004, 504, 43–52. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, X.; Liu, F.; Liu, C.; Peng, Q.; Qiao, X. Theoretical design and preparation of ionic liquid-based magnetic nanoparticles for the magnetic dispersive solid-phase extraction of benzimidazoles in human plasma. Sep. Purif. Technol. 2022, 302, 122150. [Google Scholar] [CrossRef]
- Hadavifar, M.; Bahramifar, N.; Younesi, H.; Li, Q. Adsorption of mercury ions from synthetic and real wastewater aqueous solution by functionalized multi-walled carbon nanotube with both amino and thiolated groups. Chem. Eng. J. 2014, 237, 217–228. [Google Scholar] [CrossRef]
- Guo, Q.W.; Ma, Q.Q.; Xue, Z.H.; Gao, X.D.; Chen, H.X. Studies on the binding characteristics of three polysaccharides with different molecular weight and flavonoids from corn silk. Carbohydr. Polym. 2018, 198, 581–588. [Google Scholar] [CrossRef]
- Guo, X.; Tian, Y.; Zhang, M.; Li, Y.; Wen, R.; Li, X.; Li, X.; Xue, Y.; Ma, L.; Xia, C.; et al. Mechanistic Insight into Hydrogen-Bond- Controlled Crystallinity and Adsorption Property of Covalent Organic Frameworks from Flexible Building Blocks. Chem. Mater. 2018, 30, 2299–2308. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Ma, S.; Zhang, H.; Ou, J.; Wei, Y.; Ye, M. Fabrication of Hydrazone-Linked Covalent Organic Frameworks Using Alkyl Amine as Building Block for High Adsorption Capacity of Metal Ions. ACS Appl. Mater. Interfaces 2019, 11, 11706–11714. [Google Scholar] [CrossRef]
- Sangthong, S.; Weerapreeyakul, N. Simultaneous quantification of sulforaphene and sulforaphane by reverse phase HPLC and their content in Raphanus sativus L. var. caudatus Alef extracts. Food Chem. 2016, 201, 139–144. [Google Scholar] [CrossRef]
- Han, D.; Row, K.H. Separation and Purification of Sulforaphane from Broccoli by Solid Phase Extraction. Int. J. Mol. Sci. 2011, 12, 1854–1861. [Google Scholar] [CrossRef]
- Liang, H.; Yuan, Q.P.; Dong, H.R.; Liu, Y.M. Determination of sulforaphane in broccoli and cabbage by high-performance liquid chromatography. J. Food Compos. Anal. 2006, 19, 473–476. [Google Scholar] [CrossRef]
- Hafezian, S.M.; Azizi, S.N.; Biparva, P.; Bekhradnia, A. High-efficiency purification of sulforaphane from the broccoli extract by nanostructured SBA-15 silica using solid-phase extraction method. J. Chromatogr. B Anal. Technol. Biomed Life Sci. 2019, 1108, 1–10. [Google Scholar] [CrossRef]
- Azizi, S.N.; Amiri-Besheli, B.; Sharifi-Mehr, S. The Isolation and Determination of Sulforaphane from Broccoli Tissues by Reverse Phase-High Performance Liquid Chromatography. J. Chin. Chem. Soc. 2011, 58, 906–910. [Google Scholar] [CrossRef]
- Campas-Baypoli, O.N.; Sanchez-Machado, D.I.; Bueno-Solano, C.; Ramirez-Wong, B.; Lopez-Cervantes, J. HPLC method validation for measurement of sulforaphane level in broccoli by-products. Biomed. Chromatogr. 2010, 24, 387–392. [Google Scholar] [CrossRef]
- Celik, H.; Ariburnu, E.; Baymak, M.S.; Yesilada, E. A rapid validated HPLC method for determination of sulforaphane and glucoraphanin in broccoli and red cabbage prepared by various cooking techniques. Anal. Methods 2014, 6, 4559–4566. [Google Scholar] [CrossRef]
- Ares, A.M.; Bernal, J.; Martín, M.T.; Bernal, J.L.; Nozal, M.J. Optimized Formation, Extraction, and Determination of Sulforaphane in Broccoli by Liquid Chromatography with Diode Array Detection. Food Anal. Methods 2013, 7, 730–740. [Google Scholar] [CrossRef]







| C0 (mg/L) | T (K) | ΔH (kJ/mol) | ΔS (J mol−1 K−1) | |||||
|---|---|---|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | ||||||
| Kd | ΔG (kJ/mol) | Kd | ΔG (kJ/mol) | Kd | ΔG (kJ/mol) | |||
| 1 | 2769.1 | −19.64 | 2024.1 | −19.49 | 1109.2 | −18.54 | −35.91 | −54.16 |
| 10 | 2003.3 | −18.84 | 1320.2 | −18.40 | 874.4 | −17.91 | −32.65 | −46.33 |
| 100 | 393.3 | −14.80 | 338.6 | −14.92 | 302.9 | −15.11 | −10.31 | 15.05 |
| Matrix | Linear Range (mg/L) | Regression Equation | R2 | LOD (μg/L) | LOQ (μg/L) | ME | |
|---|---|---|---|---|---|---|---|
| Before MSPE | After MSPE | ||||||
| MeOH | 0.001–0.5 | Y = 47,437,718x + 333,257 | 0.9976 | 0.035 | 0.12 | — | |
| Pak choi | 0.005–0.5 | Y = 41,538,858x + 187,6076 | 0.9951 | 0.092 | 0.31 | 0.74 | 0.88 |
| Broccoli | 0.001–0.5 | Y = 45,794,584x + 231,5048 | 0.9923 | 0.057 | 0.19 | 0.83 | 0.97 |
| Cabbage | 0.001–0.5 | Y = 42,573,695x + 3,863,071 | 0.9962 | 0.052 | 0.18 | 0.71 | 0.90 |
| Radish | 0.001–0.5 | Y = 39,017,313x + 6,545,277 | 0.9985 | 0.073 | 0.24 | 0.52 | 0.82 |
| Matrix | Original (mg kg−1) | Spiked (mg kg−1) | Recovery/% | RSD/% |
|---|---|---|---|---|
| Pak choi | 0.14 | 0.1 | 90.0 | 4.5 |
| 0.2 | 91.6 | 4.5 | ||
| 0.4 | 93.6 | 1.8 | ||
| Broccoli | 45.62 | 20 | 92.6 | 3.4 |
| 40 | 96.2 | 3.5 | ||
| 80 | 94.2 | 3.9 | ||
| Cabbage | 3.28 | 2.5 | 91.2 | 4.6 |
| 5 | 82.2 | 3.8 | ||
| 10 | 88.1 | 3.1 | ||
| Radish | 7.38 | 5 | 92.5 | 7.9 |
| 10 | 89.3 | 3.6 | ||
| 20 | 95.1 | 2.7 |
| Sample | Methods | Extraction Time or Frequency | Solvents | Linear Range (mg/L) | Recovery (%) | RSD (%) | LOD (μg/L) | LOQ (μg/L) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Pak choi, Broccoli, Cabbage, Radish | MSPE-HPLC-MS/MS | 7.5 min | 4 mL MeOH | 0.001–0.5 | 82.2–96.2 | 1.8–7.9 | 0.035–0.092 | 0.18–0.31 | This work |
| Raphanus sativus L. var. caudatus Alef | LLE-HPLC-DAD | 3 times | DCM | 5–40 | 96.8 | 0.51 | 360 | 1080 | [57] |
| Broccoli | SPE-HPLC-UV | 3 times | 24 mL DCM, 4 mL MeOH and 4 mL ethyl acetate | 0.05–200 | 90.8–96.4 | <3.6 | 20 | / | [58] |
| Broccoli and Cabbage | LLE-HPLC-DAD | 2 times | 50 mL DCM | 2.5–17.5 | 95.6 | 1.2 | / | / | [59] |
| Broccoli | LLE-UPLC–MS/MS | 3 times | 50 mL DCM | 1–10 | / | / | 77 | 235 | [25] |
| Broccoli | SPE-HPLC-UV | 3 times | 60 mL DCM | 0.3–250 | 98.4 | 1.38 | / | / | [60] |
| Brassicaceae vegetables | DLLME- HPLC-DAD | / | 1 mL ACN and 0.7 mL chloroform | 5–100 | 80–110 | <15 | 100–220 | 300–740 | [4] |
| Broccoli | HPLC-UV | 3 times | 100 mL DCM | 50–400 | >96 | / | / | / | [61] |
| Broccoli by-products | SPE-HPLC-UV | >3 h | 20 mL DCM | 4–80 | 97.5–98.1 | 3–4 | 580 | / | [62] |
| Broccoli and red cabbage | LLE-HPLC-DAD | / | 800 mL DCM | 0.09–0.36 | 95.1 | 3.8 | 29.7 | 90 | [63] |
| Broccoli | LLE-HPLC-DAD | / | 25 mL methyl t-butyl ether | 0.6–200 | 92–102 | 4–5 | 200 | 600 | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Xu, D.; Cao, J.; Shi, W.; Zhang, X.; Lin, H.; Yin, C.; Li, L.; Xu, D.; Liu, G. Facile Preparation of Magnetic COF-on-COF for Rapid Adsorption and Determination of Sulforaphane from Cruciferous Vegetables. Foods 2024, 13, 409. https://doi.org/10.3390/foods13030409
Zhou J, Xu D, Cao J, Shi W, Zhang X, Lin H, Yin C, Li L, Xu D, Liu G. Facile Preparation of Magnetic COF-on-COF for Rapid Adsorption and Determination of Sulforaphane from Cruciferous Vegetables. Foods. 2024; 13(3):409. https://doi.org/10.3390/foods13030409
Chicago/Turabian StyleZhou, Jie, Dan Xu, Jiayong Cao, Weiye Shi, Xuan Zhang, Huan Lin, Chen Yin, Lingyun Li, Donghui Xu, and Guangyang Liu. 2024. "Facile Preparation of Magnetic COF-on-COF for Rapid Adsorption and Determination of Sulforaphane from Cruciferous Vegetables" Foods 13, no. 3: 409. https://doi.org/10.3390/foods13030409