A Novel Approach to Serving Plant-Based Confectionery—The Employment of Spray Drying in the Production of Carboxymethyl Cellulose-Based Delivery Systems Enriched with Teucrium montanum L. Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
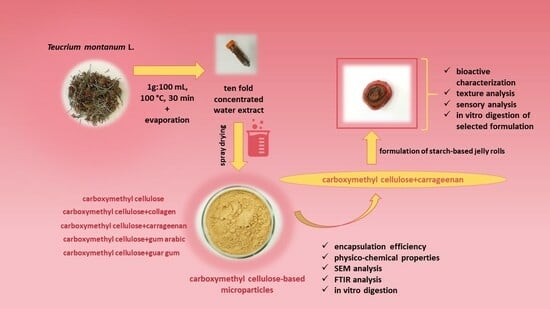
2.1.1. Extract Production and Spray-Drying Encapsulation
2.1.2. Development of Jelly Rolls
2.1.3. Chemicals and Reagents
2.2. Bioactive Characterization of Extract
2.2.1. Preparation of Polyphenolic Extract
2.2.2. Determination of Total Hydroxycinnamic Acid Derivatives (HC)
2.2.3. HPLC-UV-DAD Quantification of Phenylethanoid Glycosides (PGs)
2.3. Spray-Drying Encapsulation of Extract
2.4. Physicochemical Characterization of Powders
2.4.1. Determination of Dry Matter
2.4.2. Rheological Characterization of Feed Solutions
2.4.3. Process Yield, Encapsulation Efficiency, and Loading Capacity
2.4.4. Mean Particle Size, Zeta Potential, and Polydispersity Index (PI)
2.4.5. Wettability
2.4.6. SEM Analysis of Powders
2.4.7. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR)
2.5. In Vitro Digestion of Prepared Powders
2.6. Formulation of Starch-Based Jelly Rolls
2.7. Determination of Amylose/Amylopectin Content in Starches
2.8. Determination of Dry Matter in the Jelly Rolls
2.9. Bioactive Evaluation of Formulated Jelly Rolls
2.9.1. Extraction of PGs and Methylxanthines
2.9.2. HPLC-UV-DAD Analysis
2.10. Texture Analysis
2.11. Sensory Analysis
2.12. In Vitro Digestion of Jelly Roll
2.13. Statistical Analysis
3. Results and Discussion
3.1. Determination of HC and Phenolic Compounds in Extract
3.2. Rheological Characterization of Feed Solutions
3.3. Physicochemical Characterization of Powders
3.3.1. Process Yield, Encapsulation Efficiency, and Loading Capacity
3.3.2. SEM Analysis of Spray-Dried Powders
3.3.3. Zeta Potential, Polydispersity Index (PI), and Mean Particle Size
3.3.4. Wettability
3.3.5. ATR-FTIR
3.4. In Vitro Digestion of Microparticles
3.5. Characterization of Formulated Jelly Rolls
3.5.1. Bioactive Potential
3.5.2. Texture Profile Analysis (TPA)
3.5.3. Sensory Evaluation
3.5.4. In Vitro Digestion of Fortified Jelly Roll
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fatiha, B.A.; Ouafae, B.; Souad, S.; Fatima, E.H.; Jamila, D.; Allal, D.; Lahcen, Z. Ethnobotany study of medicinal plants used in the treatment of respiratory diseases in the middle region of Oum Rbai. Int. J. Environ. Agric. Biotechnol. 2017, 2, 1460–1468. [Google Scholar] [CrossRef]
- Zlatković, B.; Bogosavljević, S.; Radivojević, A.; Pavlović, M. Traditional use of the native medicinal plant resource of Mt. Rtanj (Eastern Serbia): Ethnobotanical evaluation and comparison. J. Ethnopharmacol. 2014, 151, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Savić, J.; Mačukanović-Jocić, M.; Jarić, S. Medical ethnobotany on the Javor mountain (Bosnia and Herzegovina). Eur. J. Integr. Med. 2019, 27, 52–64. [Google Scholar] [CrossRef]
- Šarić-Kundalić, B.; Fritz, E.; Dobeš, C.; Saukel, J. Traditional medicine in the Pristine village of Prokoško lake on Vranica mountain, Bosnia and Herzegovina. Sci. Pharm. 2010, 78, 75–290. [Google Scholar] [CrossRef] [PubMed]
- Olcha, P.; Winiarska-Mieczan, A.; Kwiecień, M.; Nowakowski, Ł.; Miturski, A.; Semczuk, A.; Kiczorowska, B.; Gałczyński, K. Antioxidative, anti-inflammatory, anti-obesogenic, and antidiabetic properties of tea polyphenols-the positive impact of regular tea consumption as an element of prophylaxis and pharmacotherapy support in endometrial cancer. Int. J. Mol. Sci. 2022, 23, 6703. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Vidal, D.-R.; Martorell, P.; Plaza, M.; Marina, M.-L. Composition of non extractable polyphenols from sweet cherry pomace determined by DART-Orbitrap-HRMS and their in vitro and in vivo potential antioxidant, antiaging, and neuroprotective activities. J. Agric. Food Chem. 2022, 70, 7993–8009. [Google Scholar] [CrossRef]
- Pinto, D.; Almeida, A.; López-Yerena, A.; Pinto, S.; Sarmento, B.; Lamuela-Raventós, R.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Appraisal of a new potential antioxidants-rich nutraceutical ingredient from chestnut shells through in-vivo assays—A targeted metabolomic approach in phenolic compounds. Food Chem. 2023, 404 Pt A, 134546. [Google Scholar] [CrossRef]
- Mitreski, I.; Petreska Stanoeva, J.; Stefova, M.; Stefkov, G.; Kulevanova, S. Polyphenols in representative Teucrium species in the flora of R. Macedonia: LC/DAD/ESI-MSn profile and content. Nat. Prod. Commun. 2014, 9, 175–180. [Google Scholar] [CrossRef]
- Nastić, N.; Švarc-Gajića, J.; Delerue-Matos, C.; Barroso, M.F.; Soares, C.; Moreira, M.M.; Morais, S.; Mašković, P.; Gaurina Srček, V.; Slivac, I.; et al. Subcritical water extraction as an environmentally-friendly technique to recover bioactive compounds from traditional Serbian medicinal plants. Ind. Crop. Prod. 2018, 111, 579–589. [Google Scholar] [CrossRef]
- Bektašević, M.; Jurin, M.; Roje, M.; Politeo, O. Phytochemical profile, antioxidant activity and cholinesterase inhibition potential of essential oil and extracts of Teucrium montanum from Bosnia and Herzegovina. Separations 2023, 10, 421. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.; Pan, J.; Ma, K. Verbascoside: A neuroprotective phenylethanoid glycosides with anti-depressive properties. Phytomedicine 2023, 120, 155027. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, Z.; Chen, P.; Zhao, Z.; Lin, C.; Zhu, C.; Wu, A. Insights on the interactions of human serum albumin with three natural phenylethanoid glycosides that inhibit HeLa cells proliferation. J. Mol. Struct. 2022, 1251, 132050. [Google Scholar] [CrossRef]
- Abudujilile, D.; Wang, W.; Aimaier, A.; Chang, L.; Dong, Y.; Wang, Y.; Fan, X.; Ma, Y.; Wang, Y.; Ziyayiding, D.; et al. Cistanche tubulosa phenylethanoid glycosides suppressed adipogenesis in 3T3-L1 adipocytes and improved obesity and insulin resistance in high-fat diet induced obese mice. BMC Complement. Altern. Med. 2022, 22, 270. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Li, X.; Gao, B.; Zhang, C.; Li, M. Phenylethanoid glycosides from traditional Mongolian medicine Cymbaria daurica alleviate alloxan-induced INS-1 cells oxidative stress and apoptosis. Food Sci. Hum. Wellness 2023, 12, 1580–1598. [Google Scholar] [CrossRef]
- Okasha, Y.M.; Fathy, F.I.; Fathy, M.S.; Fayek, N.M. The untargeted phytochemical profile of Verbascum thapsus L. with potent antiviral, antibacterial and anticancer activities. S. Afr. J. Bot. 2023, 156, 334–341. [Google Scholar] [CrossRef]
- Hu, K.; Guan, W.J.; Bi, Y.; Zhang, W.; Li, L.; Zhang, B.; Liu, Q.; Song, Y.; Li, X.; Duan, Z.; et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine 2021, 85, 153242. [Google Scholar] [CrossRef] [PubMed]
- de Araujo Ribas Cheohen, C.-F.; Alves Esteves, M.E.; da Fonseca, T.S.; Monteiro Leal, C.; de Lemos Fernandes Assis, F.; Freire Campos, M.; Soares Rebelo, R.; Allonso, D.; Guimarães Leitão, G.; da Silva Leal, M.; et al. In silico screening of phenylethanoid glycosides, a class of pharmacologically active compounds as natural inhibitors of SARS-CoV-2 proteases. Comput. Struct. Biotechnol. J. 2023, 21, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M.; et al. Available technologies on improving the stability of polyphenols in food processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- Mérillon, J.M.; Ramawat, K.G. Bioactive Molecules in Food; Springer International Publishing: New York, NY, USA, 2019; p. 2370. [Google Scholar]
- Hoyos-Leyva, J.D.; Bello-Pérez, L.A.; Alvarez-Ramirez, J.; Garcia, H.S. Microencapsulation using starch as wall material: A review. Food Rev. Int. 2018, 34, 148–161. [Google Scholar] [CrossRef]
- Zuidam, N.J.; Nedovic, V. Encapsulation Technologies for Active Food Ingredients and Food Processing; Springer Science + Business Media: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Etzbach, L.; Meinert, M.; Faber, T.; Klein, C.; Schieber, A.; Weber, F. Effects of carrier agents on powder properties, stability of carotenoids, and encapsulation efficiency of goldenberry (Physalis peruviana L.) powder produced by co-current spray drying. Curr. Res. Food Sci. 2020, 3, 73–81. [Google Scholar] [CrossRef]
- Grace, M.H.; Hoskin, R.; Xiong, J.; Lila, M.A. Whey and soy proteins as wall materials for spray drying rosemary: Effects on polyphenol composition, antioxidant activity, bioaccessibility after in vitro gastrointestinal digestion and stability during storage. LWT-Food Sci. Technol. 2021, 149, 111901. [Google Scholar] [CrossRef]
- Ricci, A.; Arboleda Mejia, J.A.; Versari, A.; Chiarello, E.; Bordoni, A.; Parpinello, G.P. Microencapsulation of polyphenolic compounds recovered from red wine lees: Process optimization and nutraceutical study. Food Bioprod. Process. 2022, 132, 1–12. [Google Scholar] [CrossRef]
- Ravichandran, K.S.; Silva, E.S.; Moncada, M.; Perkins-Veazie, P.; Lila, M.A.; Greenlief, C.M.; Thomas, A.L.; Hoskin, R.T.; Krishanswamy, K. Spray drying to produce novel phytochemical-rich ingredients from juice and pomace of American elderberry. Food Biosci. 2023, 55, 102981. [Google Scholar] [CrossRef]
- Nguyen, Q.-D.; Dang, T.-T.; Nguyen, T.-V.-L.; Nguyen, T.-T.-D.; Nguyen, N.-N. Nguyen. Microencapsulation of roselle (Hibiscus sabdariffa L.) anthocyanins: Effects of different carriers on selected physicochemical properties and antioxidant activities of spray-dried and freeze-dried powder. Int. J. Food Prop. 2022, 25, 359–374. [Google Scholar] [CrossRef]
- Tezuka, Y.; Tsuchiya, Y.; Shiomi, T. 13C NMR determination of substituent distribution in carboxymethylcellulose by use of its peresterified derivatives. Carbohydr. Polym. 1996, 291, 99–108. [Google Scholar] [CrossRef]
- Bifani, V.; Ramírez, C.; Ihl, M.; Rubilar, M.; García, A.; Zaritzky, N. Effects of murta (Ugni molinae Turcz) extract on gas and water vapor permeability of carboxymethylcellulose-based edible films. LWT-Food Sci. Technol. 2007, 40, 1473–1481. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Production and physicochemical properties of carboxymethyl cellulose films enriched with spent coffee grounds polysaccharides. Int. J. Biol. Macromol. 2018, 106, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Mandura Jarić, A.; Čikoš, A.; Pocrnić, M.; Aladić, K.; Jokić, S.; Šeremet, D.; Vojvodić Cebin, A.; Komes, D. Teucrium montanum L.—Unrecognized Source of Phenylethanoid Glycosides: Green Extraction Approach and Elucidation of Phenolic Compounds via NMR and UHPLC-HR MS/MS. Antioxidants 2023, 12, 1903. [Google Scholar] [CrossRef]
- MarkWide Research. Global Mochi Market Analysis. 2023. Available online: https://markwideresearch.com/global-mochi-market/ (accessed on 11 November 2023).
- Bharti, R.; Chopra, B.S.; Raut, S.; Khatri, N. Pueraria tuberosa: A Review on Traditional Uses, Pharmacology, and Phytochemistry. Front. Pharmacol. 2021, 11, 582506. [Google Scholar] [CrossRef]
- Imamoglu, H.; Coggins, P.C.; Rowe, D.E. Influence of storage time and starches on texture attributes of conventional milk yogurt using response surface methodology. Int. Food Res. J. 2017, 24, 1721–1727. [Google Scholar]
- Reddy, C.K.; Luan, F.; Xu, B. Morphology, crystallinity, pasting, thermal and quality characteristics of starches from adzuki bean (Vigna angularis L.) and edible kudzu (Pueraria thomsonii Benth). Int. J. Biol. Macromol. 2017, 105, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Mandura Jarić, A.; Šeremet, D.; Vojvodić Cebin, A.; Jokić, S.; Komes, D. The multiple-response modeling of heat-assisted, microwave-assisted and subcritical water extraction on selected phenolics from traditional plant species Teucrium montanum. Prep. Biochem. Biotechnol. 2022, 52, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Matkowski, A.; Tasarz, P.; Szypuła, E. Antioxidant activity of herb extracts from five medicinal plants from Lamiaceae, subfamily Lamioideae. J. Med. Plant Res. 2008, 2, 321–330. [Google Scholar]
- Padmore, J.M. Animal feed—AOAC official method 930.15—Moisture in animal feed. In Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; AOAC International: Arlington, VA, USA, 1990; Volume 1, pp. 69–70. [Google Scholar]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Adamson, G.E.; Lazarus, S.A.; Mitchell, A.E.; Prior, R.L.; Cao, G.; Jacobs, P.H.; Kremers, B.G.; Hammerstone, J.F.; Rucker, R.B.; Ritter, K.A.; et al. HPLC method for the quantification of procyanidins in cocoa and chocolate samples and correlation to total antioxidant capacity. J. Agric. Food Chem. 1999, 47, 4184–4188. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Sensory Analysis—Identification and Selection of Descriptors for Establishing a Sensory Profile by a Multidimensional Approach (ISO 11035:1994). 1994. Available online: https://www.iso.org/standard/19015.html (accessed on 2 November 2023).
- Saint-Eve, A.; Déléris, I.; Panouillé, M.; Dakowski, F.; Cordelle, S.; Schlich, P.; Souchon, I. How Texture Influences Aroma and Taste Perception Over Time in Candies. Chemosens. Percept. 2011, 4, 32–41. [Google Scholar] [CrossRef]
- Kartbaeva, E.B.; Donald, G.R.; Sakipova, Z.B.; Ibragimova, L.N.; Bekbolatova, E.N.; Ternynko, I.I.; Boylan, F. Antinociceptive activity of Cistanche salsa stolons, growing in the Republic of Kazakhstan. Rev. Bras. Farmacogn. 2017, 27, 587–591. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, J.; Yang, Z.; Liu, H.; Li, S.; Wu, B.; Ma, Z. Quantitative analysis of Cistanches Herba using high-performance liquid chromatography coupled with diode array detection and high-resolution mass spectrometry combined with chemometric methods. J. Sep. Sci. 2013, 36, 1945–1952. [Google Scholar] [CrossRef]
- Esposito, T.; Sansone, F.; Auriemma, G.; Franceschelli, S.; Pecoraro, M.; Picerno, P.; Aquino, R.P.; Mencherini, T. Study on Ajuga reptans extract: A natural antioxidant in microencapsulated powder form as an active ingredient for nutraceutical or pharmaceutical purposes. Pharmaceutics 2020, 12, 671. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Nishinari, K.; Zhang, H. Rheological study of gum arabic solutions: Interpretation based on molecular self-association. Food Hydrocoll. 2009, 23, 2394–2402. [Google Scholar] [CrossRef]
- Dong, S.; Feng, S.; Liu, F.; Li, R.; Li, W.; Liu, F.; Shi, G.; Chen, L.; Zhang, Y. Factors influencing the adhesive behavior of carboxymethyl cellulose-based hydrogel for food applications. Int. J. Biol. Macromol. 2021, 179, 398–406. [Google Scholar] [CrossRef]
- Williams, P.A.; Phillips, G.O. Gum arabic. In Handbook of Hydrocolloids; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing Series in Food Science; Technology and Nutrition: Sawston, UK, 2009; pp. 252–273. [Google Scholar]
- Wyatt, N.B.; Gunther, C.M.; Liberatore, M.W. Increasing viscosity in entangled polyelectrolyte solutions by the addition of salt. Polymer 2011, 52, 2437–2444. [Google Scholar] [CrossRef]
- Bai, L.; Liu, F.; Xu, X.; Huan, S.; Gu, J.; McClements, D.J. Impact of polysaccharide molecular characteristics on viscosity enhancement and depletion flocculation. J. Food Eng. 2017, 207, 35–45. [Google Scholar] [CrossRef]
- Razmkhah, S.; Razavi, S.M.A.; Mohammadifar, M.A. Dilute solution, flow behavior, thixotropy and viscoelastic characterization of cress seed (Lepidium sativum) gum fractions. Food Hydrocoll. 2017, 63, 404–413. [Google Scholar] [CrossRef]
- Amirabadi, S.; Milani, J.M.; Sohbatzadeh, F. Effects of cold atmospheric-pressure plasma on the rheological properties of gum Arabic. Food Hydrocol. 2021, 117, 106724. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook for Users of Rotational and Oscillatory Rheometers; Vincentz Network GmbH & Co. KG: Hannover, Germany, 2006. [Google Scholar]
- Steffe, J.F. Rheological Methods in Food Process Engineering; Freeman Press: Dallas, TX, USA, 1996. [Google Scholar]
- Simões, A.; Miranda, M.; Cardoso, C.; Veiga, F.; Vitorino, C. Rheology by design: A regulatory tutorial for analytical method validation. Pharmaceutics 2020, 12, 820. [Google Scholar] [CrossRef] [PubMed]
- Castro-López, C.; Espinoza-González, C.; Ramos-González, R.; Boone-Villa, V.D.; Aguilar-González, M.G.; Martínez-Ávila, G.C.G.; Aguilar, C.N.; Ventura-Sobrevilla, J.M. Spray-drying encapsulation of microwave-assisted extracted polyphenols from Moringa oleifera: Influence of tragacanth, locust bean, and carboxymethyl-cellulose formulations. Food Res. Int. 2021, 144, 110291. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, B.R.; Datta, N.; Crooks, R.; Howes, T.; Rigby, S. A semi-empirical approach to optimize the quantity of drying aids required to spray dry sugar rich foods. Dry. Technol. 1997, 15, 2509–2525. [Google Scholar] [CrossRef]
- Boonyai, P.; Bhandari, B.; Howes, T. Stickiness measurement techniques for food powders: A review. Powder Technol. 2004, 145, 34–46. [Google Scholar] [CrossRef]
- Ross, Y.H. The glassy state. In Food Materials Science: Principles and Practice; Lillford, P., Aguilera, J.M., Eds.; Springer: New York, NY, USA, 2008; pp. 67–81. [Google Scholar]
- Jaya, S.; Das, H. Glass transition and sticky point temperatures and stability/mobility diagram of fruit powders. Food Bioprocess Technol. 2008, 2, 89–95. [Google Scholar] [CrossRef]
- Woo, M.; Daud, W.; Mujumdar, A.; Tasirin, S.; Talib, M. Role of rheological characteristics in amorphous food particle-wall collisions in spray drying. Powder Technol. 2010, 198, 251–257. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Lozano-Sánchez, J.; Cádiz-Gurrea, M.d.l.L.; Fernández-Ochoa, Á.; Arráez-Román, D.; Segura-Carretero, A. Spray-drying microencapsulation of bioactive compounds from Lemon Verbena green extract. Foods 2020, 9, 1547. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.A.; Jarafi, S.M.; Assadpoor, E.; Dehnad, D. Microencapsulation optimization of natural anthocyanins with maltodextrin, gum arabic and gelatin. Int. J. Biol. Macromol. 2016, 85, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Le Bourvellec, C.; Renard, M.G.C.C. Interactions between cell wall polysaccharides and polyphenols: Effect of molecular internal structure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3574–3617. [Google Scholar] [CrossRef] [PubMed]
- Dhanalakshmi, K.; Ghosal, S.; Bhattacharya, S. Agglomeration of food powder and applications. Crit. Rev. Food Sci. Nutr. 2011, 51, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.I.; Rodrigues, M.Z.; Pinto, M.R.M.R.; Vanzela, E.S.L.; Stringheta, P.C.; Perrone, Í.T.; Ramos, A.M. Morphological characterization of pequi extract microencapsulated through spray drying. Int. J. Food Prop. 2017, 20, 1298–1305. [Google Scholar] [CrossRef]
- Kinalski, T.; Noreña, C.P.Z. Effect of spray drying encapsulation of garlic extract on inulin and thiosulfinates contents. J. Food Meas. Charact. 2019, 13, 2438–2447. [Google Scholar] [CrossRef]
- Vargas-Muñoz, D.P.; da Silva, L.C.; de Oliveira, L.A.N.; Godoy, H.R.; Kurozawa, L.E. 5-caffeoylquinic acid retention in spray drying of cocona, an Amazonian fruit, using hydrolyzed collagen and maltodextrin as encapsulating agents. Dry. Technol. 2021, 39, 1854–1868. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of pineapple peel extract by spray drying using maltodextrin, inulin, and gum arabic as wall matrices. Foods 2020, 9, 718. [Google Scholar] [CrossRef]
- Cabral, B.R.P.; de Oliveira, P.M.; Gelfuso, G.M.; Quintão, T.D.S.C.; Chaker, J.A.; Karnikowski, M.G.D.O.; Gris, E.F. Improving stability of antioxidant compounds from Plinia cauliflora (jabuticaba) fruit peel extract by encapsulation in chitosan microparticles. J. Food Eng. 2018, 238, 195–201. [Google Scholar] [CrossRef]
- Souza, F.N.; Gebara, C.; Ribeiro, M.C.; Chaves, K.S.; Gigante, M.L.; Grosso, C.R. Production and characterization of microparticles containing pectin and whey proteins. Food Res. Int. 2012, 49, 560–566. [Google Scholar] [CrossRef]
- Hashmi, M.; Ullah, S.; Ullah, A.; Saito, Y.; Haider, M.K.; Bie, X.; Wada, K.; Kim, I.S. Carboxymethyl Cellulose (CMC) based electrospun composite nanofiber mats for food packaging. Polymers 2021, 13, 302. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qin, S.; Xie, J.; Lin, X.; Zheng, Y.; Yang, J.; Kan, H.; Shi, Z. Using Carboxymethyl Cellulose as the Additive with Enzyme-Catalyzed Carboxylated Starch to Prepare the Film with Enhanced Mechanical and Hydrophobic Properties. Front. Bioeng. Biotechnol. 2021, 9, 638546. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, H.; Dehghani, M.; Miri, S.; Pazirofteh, M.; Garnier, G.; Batchelor, W. Highly hydrophobic and moisture barrier nanocellulose based films produced via spray deposition. Cellulose 2023, 30, 5157–5170. [Google Scholar] [CrossRef]
- Cuba-Chiem, L.T.; Huynh, L.; Ralston, J.; Beattie, D.A. In situ particle film ATR FTIR spectroscopy of carboxymethyl cellulose adsorption on talc: Binding mechanism, pH effects, and adsorption kinetics. Langmuir 2008, 24, 8036–8044. [Google Scholar] [CrossRef]
- Emam, H.E. Gum arabic as bio-synthesizer for ag–au bimetallic nanocomposite using seed-mediated growth technique and its biological efficacy. J. Polym. Environ. 2019, 27, 210–223. [Google Scholar] [CrossRef]
- Moghadam, A.; Mobarakeh, M.S.; Safaei, M.; Kariminia, S. Synthesis and characterization of novel bio-nanocomposite of polyvinyl alcohol-Gum arabic-magnesium oxide via direct blending method. Carbohydr. Polym. 2021, 260, 117802. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D.; Barak, S.; Khatkar, B.S. X-ray diffraction, IR spectroscopy and thermal characterization of partially hydrolyzed guar gum. Int. J. Biol. Macromol. 2012, 50, 1035–1039. [Google Scholar] [CrossRef]
- Anjum, A.; Zuber, M.; Zia, K.M.; Anjum, M.N.; Aftab, W. Preparation and characterization of guar gum based polyurethanes. Int. J. Biol. Macromol. 2021, 183, 2174–2183. [Google Scholar] [CrossRef]
- Ficai, A.; Albu, M.G.; Birsan, M.; Sonmez, M.; Ficai, D.A.; Trandafir, V.; Andronescu, E. Collagen hydrolysate based collagen/hydroxyapatite composite materials. J. Mol. Struct. 2013, 1037, 154–159. [Google Scholar] [CrossRef]
- Chopin, T.; Kerin, B.F.; Mazerolle, R. Phycocolloid chemistry as a taxonomic indicator of phylogeny in the Gigartinales, Rhodophyceae: A review and current developments using Fourier transform infrared diffuse reflectance spectroscopy. Phycol. Res. 1999, 4, 167–188. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, S.; Chen, P.; Liu, Z.; Lin, L.; Zhang, J. Effect of physiological pH on the molecular characteristics, rheological behavior, and molecular dynamics of κ-carrageenan/casein. Front. Nutr. 2023, 10, 1174888. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Wang, W.; Song, Y.; Ou, Y.; Zhu, J. Structural and physical properties of carboxymethyl cellulose/gelatin films functionalized with antioxidant of bamboo leaves. Int. J. Biol. Macromol. 2020, 164, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Amoako, D.; Awika, J.M. Polyphenol interaction with food carbohydrates and consequences on availability of dietary glucose. Curr. Opin. Food Sci. 2016, 8, 14–18. [Google Scholar] [CrossRef]
- Dobson, C.C.; Mottawea, W.; Rodrigue, A.; Buzati Pereira, B.L.; Hammami, R.; Power, K.A.; Bordenave, N. Chapter Three- Impact of molecular interactions with phenolic compounds on food polysaccharides functionality. In Advances in Food and Nutrition Research; Ferreira, I.C.F.R., Barros, L., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 90, Chapter 3; pp. 135–181. [Google Scholar] [CrossRef]
- Wei, X.; Dai, J.; Zhong, Y.; Zhang, D.; Liu, L.; Wang, L.; Huang, Y.; Chen, P.; Zhou, Z.; Chen, X.; et al. Caffeic acid phenethyl ester loaded in nano-targeted delivery system with casein: Physicochemical characterization, in vitro release, and binding mechanisms. LWT- Food Sci. Technol. 2021, 150, 111938. [Google Scholar] [CrossRef]
- Jiang, Y.; Mao, S.; Huang, W.; Lu, B.; Cai, Z.; Zhou, F.; Li, M.; Lou, T.; Zhao, Y. Phenylethanoid glycoside profiles and antioxidant activities of Osmanthus fragrans Lour. Flowers by UPLC/PDA/MS and simulated digestion model. J. Agric. Food Chem. 2016, 64, 2459–2466. [Google Scholar] [CrossRef]
- Gayoso, L.; Claerbout, A.-S.; Calvo, M.I.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D. Bioaccessibility of rutin, caffeic acid and rosmarinic acid: Influence of the in vitro gastrointestinal digestion models. J. Funct. Foods 2016, 26, 428–438. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Chen, Z.; Luo, X.; Li, Y.; Wang, R.; Li, J.; Li, Y.; Wang, T.; Wu, J. Effects of milk proteins on the bioaccessibility and antioxidant activity of oat phenolics during in vitro digestion. J. Food Sci. 2019, 84, 895–903. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Chen, W.; Wang, L. Encapsulation of caffeic acid into sodium caseinate using ph-driven method: Fabrication, characterization, and bioavailability. Food Bioprocess Technol. 2023. [Google Scholar] [CrossRef]
- Cedeño-Pinos, C.; Martínez-Tomé, M.; Jordán, M.J.; Bañón, S. Revalorisation of sage (Salvia lavandulifolia Vahl) by-product extracts as a source of polyphenol antioxidants for novel jelly candies. Antioxidants 2023, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Cedeño-Pinos, C.; Martínez-Tomé, M.; Murcia, M.A.; Jordán, M.J.; Bañón, S. Assessment of rosemary (Rosmarinus officinalis L.) extract as antioxidant in jelly candies made with fructan fibres and stevia. Antioxidants 2020, 9, 1289. [Google Scholar] [CrossRef] [PubMed]
- de Mouraa, S.C.S.R.; Berlingb, C.L.; Garciac, A.O.; Queirozd, M.B.; Alvimd, I.D.; Hubinger, M.D. Release of anthocyanins from the hibiscus extract encapsulated by ionic gelation and application of microparticles in jelly candy. Food Res. Int. 2023, 121, 542–552. [Google Scholar] [CrossRef]
- Sakulnarmrat, K.; Konczak, I. Encapsulation of Melodorum fruticosum Lour. anthocyanin-rich extract and its incorporation into model food. LWT Food Sci. Technol. 2023, 153, 112546. [Google Scholar] [CrossRef]
- Lu, S.; Cik, T.-T.; Lii, C.-Y.; Lai, P.; Chen, H.-H. Effect of amylose content on structure, texture and a-amylase reactivity of cooked rice. LWT Food Sci. Technol. 2013, 54, 224–228. [Google Scholar] [CrossRef]
- Kohyama, K.; Sodhi, N.S.; Suzuki, K.; Sasaki, T. Texture evaluation of cooked rice prepared from japanese cultivars using two-bite instrumental test and electromyography. J. Texture Stud. 2016, 47, 188–198. [Google Scholar] [CrossRef]
- Sengul, H.; Surek, E.; Nilufer-Erdil, D. Investigating the effects of food matrix and food components on bioaccessibility of pomegranate (Punica granatum) phenolics and anthocyanins using an in vitro gastrointestinal digestion model. Food Res. Int. 2014, 62, 1069–1079. [Google Scholar] [CrossRef]
- Thumann, T.A.; Pferschy-Wenzig, E.-M.; Aziz-Kalbhenn, H.; Ammar, R.M.; Rabini, S.; Moissl-Eichinger, C.; Bauer, R. Application of an in vitro digestion model to study the metabolic profile changes of an herbal extract combination by UHPLC-HRMS. Phytomedicine 2020, 71, 153221. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, F.; Peng, X.; Cheng, K.; Xiao, L.; Zhang, H.; Li, H.; Jiang, L.; Deng, Z. Metabolism of phenolics of Tetrastigma hemsleyanum roots under in vitro digestion and colonic fermentation as well as their in vivo antioxidant activity in rats. Foods 2021, 10, 2123. [Google Scholar] [CrossRef]
- Takahama, U.; Hirota, S. Effects of starch on nitrous acid-induced oxidation of kaempferol and inhibition of α-amylase-catalysed digestion of starch by kaempferol under conditions simulating the stomach and the intestine. Food Chem. 2013, 141, 313–319. [Google Scholar] [CrossRef]
- Bahloul, N.; Bellili, S.; Aazza, S.; Chérif, A.; Faleiro, M.L.; Antunes, M.D.; Miguel, M.G.; Mnif, W. Aqueous extracts from Tunisian Diplotaxis: Phenol content, antioxidant and anti-acetylcholinesterase activities, and impact of exposure to simulated gastrointestinal fluids. Antioxidants 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Kaeswurm, J.A.H.; Burandt, M.R.; Mayer, P.S.; Straub, L.V.; Buchweitz, M. Bioaccessibility of apple polyphenols from peel and flesh during oral digestion. J. Agric. Food Chem. 2022, 70, 4407–4417. [Google Scholar] [CrossRef] [PubMed]
- Hiolle, M.; Lechevalier, V.; Floury, J.; Boulier-Monthéan, N.; Prioul, C.; Dupont, D.; Nau, F. In vitro digestion of complex foods: How microstructure influences food disintegration and micronutrient bioaccessibility. Food Res. Int. 2019, 128, 108817. [Google Scholar] [CrossRef] [PubMed]








| Ingredient | 100CS | 75CS_25K | 50CS_50K | 25CS_75K | 100K |
|---|---|---|---|---|---|
| Sucrose (%) | 58 | 58 | 58 | 58 | 58 |
| Glucose syrup (%) | 19 | 19 | 19 | 19 | 19 |
| Corn starch (%) | 19 | 14 | 9.5 | 5 | / |
| Kudzu starch (%) | / | 5 | 9.5 | 14 | 19 |
| Citric acid (%) | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 |
| Raspberry flavor (%) | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Natural red color (%) | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Lyophilized raspberries (%) | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 |
| Glycerol (%) | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 |
| HC (g L−1) * | Echinacoside (g L−1) | Teupolioside (g L−1) * | Stachysoside A (g L−1) * | Poliumoside (g L−1) | Verbascoside (g L−1) |
|---|---|---|---|---|---|
| mg g−1 dw of plant | |||||
| 101.82 ± 3.66 | 23.54 ± 1.10 | 6.93 ± 0.42 | 13.39 ± 0.83 | 21.72 ± 0.22 | 7.90 ± 0.46 |
| g L−1 of concentrated extract | |||||
| 8.16 ± 0.00 | 2.38 ± 0.00 | 0.69 ± 0.00 | 1.30 ± 0.00 | 0.92 ± 0.00 | 0.60 ± 0.00 |
| Sample | G′ LVE (Pa) | G″ LVE (Pa) | tan δ LVE | η (mPa·s) |
|---|---|---|---|---|
| CMC | 2.28 ± 0.16 a | 3.95 ± 0.07 a | 1.75 ± 0.15 | 298.76 ± 1.05 a |
| CMC_CLG | 0.45 ± 0.08 a | 1.36 ± 0.06 ab | 3.16 ± 0.36 | 110.05 ± 0.66 a |
| CMC_CAR | 1.30 ± 0.32 | 2.44 ± 0.01 abc | 2.01 ± 0.51 | 168.86 ± 0.78 a |
| CMC_AG | 0.59 ± 0.13 a | 1.39 ± 0.12 ac | 2.44 ± 0.33 | 98.97 ± 0.22 a |
| CMC_GG | 0.32 ± 0.07 a | 1.01 ± 0.11 ac | 3.27 ± 0.36 | 72.08 ± 0.06 a |
| Samples | Process Yield (%) | Dry Matter (%) | Contact Angle (°) | Mean Particle Size (µm) | Zeta Potential (mV) | Polydispersity Index |
|---|---|---|---|---|---|---|
| CMC | 32.55 ± 2.15 | 94.48 ± 0.92 a | 144 ± 1.00 a | 2.93 ± 1.46 a | −15.59 ± 0.24 a | 0.95 ± 0.05 a |
| CMC_CLG | 54.48 ± 1.56 | 97.20 ± 0.11 | 105 ± 3.00 b | 3.09 ± 0.88 b | −15.41 ± 0.48 a | 0.91 ± 0.09 a |
| CMC_CAR | 55.00 ± 2.01 a | 96.92 ± 0.34 a | 107 ± 1.50 b | 2.88 ± 1.23 abc | −14.70 ± 0.35 a | 0.82 ± 0.04 a |
| CMC_AG | 53.16 ± 4.99 a | 95.94 ± 0.21 a | 140 ± 1.00 ac | 2.96 ± 1 13 bc | −17.91 ± 0.48 | 1.00 ± 0.00 a |
| CMC_GG | 47.61 ± 3.56 a | 96.27 ± 0.05 a | 136 ± 2.87 ac | 2.39 ± 0.91 a | −11.76 ± 0.35 | 0.97 ± 0.03 a |
| Encapsulation Efficiency (%) | ||||||
|---|---|---|---|---|---|---|
| Sample | HC | Echinacoside | Teupolioside | Stachysoside A | Poliumoside | Verbascoside |
| CMC | 76.85 ± 0.27 | 84.02 ± 0.20 a | 99.34 ± 0.16 ac | 88.13 ± 0.21 | 88.07 ± 0.77 a | 92.23 ± 0.83 a |
| CMC_CLG | 97.31 ± 0.31 a | 84.35 ± 0.16 a | 88.63 ± 0.38 | 83.79 ± 0.33 | 87.46 ± 0.15 a | 85.44 ± 0.07 |
| CMC_CAR | 68.67 ± 0.94 | 77.36 ± 0.50 | 80.41 ± 0.11 | 76.25 ± 0.19 | 79.51 ± 0.02 | 76.50 ± 0.25 |
| CMC_AG | 92.15 ± 2.57 ab | 90.62 ± 0.44 b | 103.31 ± 1.06 b | 94.07 ± 0.01 | 94.85 ± 0.20 b | 95.79 ± 0.49 b |
| CMC_GG | 90.12 ± 1.07 ab | 90.61 ± 0.42 b | 101.66 ± 0.07 abc | 92.71 ± 0.01 | 94.04 ± 0.02 b | 94.80 ± 0.32 ab |
| Loading capacity (%) | ||||||
| CMC | 20.15 ± 0.40 | 6.13 ± 0.01 | 1.80 ± 0.03 | 3.32 ± 0.02 | 2.37 ± 0.06 | 1.55 ± 0.01 |
| CMC_CLG | 23.12 ± 0.36 | 4.54 ± 0.01 a | 1.17 ± 0.02 | 2.33 ± 0.00 | 1.73 ± 0.01 a | 1.09 ± 0.01 a |
| CMC_CAR | 22.30 ± 0.38 | 4.37 ± 0.02 a | 1.10 ± 0.01 | 2.17 ± 0.01 | 1.65 ± 0.01 a | 0.99 ± 0.00 a |
| CMC_AG | 22.09 ± 0.08 | 4.10 ± 0.01 a | 1.18 ± 0.02 | 2.20 ± 0.01 | 1.62 ± 0.01 a | 1.01 ± 0.01 a |
| CMC_GG | 21.55 ± 0.45 | 4.21 ± 0.02 a | 1.20 ± 0.00 | 2.21 ± 0.02 | 1.61 ± 0.03 a | 1.04 ± 0.01 a |
| Sample | mg g−1 per Serving | ||||||
|---|---|---|---|---|---|---|---|
| Echinacoside | Teupolioside * | Stachysoside A * | Poliumoside * | Verbascoside | Teobromine | Caffeine | |
| 100CS | 2.27 ± 0.06 | 0.80 ± 0.01 | 0.94 ± 0.02 | 2.99 ± 0.07 | 0.85 ± 0.02 | 4.99 ± 0.11 | 0.54 ± 0.01 |
| 75CS_25K | 1.38 ± 0.01 | 0.47 ± 0.00 | 0.56 ± 0.00 | 1.81 ± 0.02 | 0.50 ± ±0.01 | 3.11 ± 0.03 | 0.33 ± 0.00 |
| 50CS_50K | 2.12 ± 0.03 | 0.70 ± 0.00 | 0.86 ± 0.02 | 2.71 ± 0.04 | 0.770.01 | 4.60 ± 0.06 | 0.49 ± 0.01 |
| 25CS_75K | 2.29 ± 0.02 | 0.76 ± 0.00 | 0.92 ± 0.01 | 2.91 ± 0.03 | 0.82 ± 0.01 | 4.92 ± 0.04 | 0.53 ± 0.01 |
| 100K | 2.73 ± 0.00 | 0.92 ± 0.01 | 1.12 ± 0.01 | 3.48 ± 0.00 | 1.01 ± 0.00 | 5.83 ± 0.01 | 0.62 ± 0.00 |
| Sample | Hardness (g) | Cohesiveness | Springiness | Chewiness | Resilience | Adhesiveness (g.s) |
|---|---|---|---|---|---|---|
| 100CS | 794.94 ± 7.42 a | 1.22 ± 0.67 | 1970.45 ± 370.45 | 35,506.30 ± 1428 | 32.29 ± 5.88 | −5.04 ± 1.26 |
| 75CS_25K | 850.78 ± 9.79 | 1.67 ± 0.14 | 2305.68 ± 26.14 | 32,855.89 ± 538 | 37.15 ± 1.79 | −10.16 ± 3.85 |
| 50CS_50K | 765.81 ± 14.40 b | 1.91 ± 0.11 | 2327.27 ± 4.55 | 34,089.32 ± 1312 | 35.55 ± 0.63 | −6.66 ± 0.78 |
| 25CS_75K | 829.46 ± 62.90 | 1.38 ± 0.45 | 2248.86 ± 87.50 | 25,446.69 ± 1408 | 34.02 ± 1.39 | −10.89 ± 6.64 |
| 100K | 978.94 ± 28.42 ab | 1.63 ± 0.03 | 2359.09 ± 2.27 | 37,701.42 ± 425 | 38.17 ± 5.20 | −3.03 ± 2.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandura Jarić, A.; Haramustek, L.; Nižić Nodilo, L.; Vrsaljko, D.; Petrović, P.; Kuzmić, S.; Jozinović, A.; Aladić, K.; Jokić, S.; Šeremet, D.; et al. A Novel Approach to Serving Plant-Based Confectionery—The Employment of Spray Drying in the Production of Carboxymethyl Cellulose-Based Delivery Systems Enriched with Teucrium montanum L. Extract. Foods 2024, 13, 372. https://doi.org/10.3390/foods13030372
Mandura Jarić A, Haramustek L, Nižić Nodilo L, Vrsaljko D, Petrović P, Kuzmić S, Jozinović A, Aladić K, Jokić S, Šeremet D, et al. A Novel Approach to Serving Plant-Based Confectionery—The Employment of Spray Drying in the Production of Carboxymethyl Cellulose-Based Delivery Systems Enriched with Teucrium montanum L. Extract. Foods. 2024; 13(3):372. https://doi.org/10.3390/foods13030372
Chicago/Turabian StyleMandura Jarić, Ana, Laura Haramustek, Laura Nižić Nodilo, Domagoj Vrsaljko, Predrag Petrović, Sunčica Kuzmić, Antun Jozinović, Krunoslav Aladić, Stela Jokić, Danijela Šeremet, and et al. 2024. "A Novel Approach to Serving Plant-Based Confectionery—The Employment of Spray Drying in the Production of Carboxymethyl Cellulose-Based Delivery Systems Enriched with Teucrium montanum L. Extract" Foods 13, no. 3: 372. https://doi.org/10.3390/foods13030372