Anti-Salmonella Activity of Thymus serpyllum Essential Oil in Sous Vide Cook–Chill Rabbit Meat
Abstract
:1. Introduction
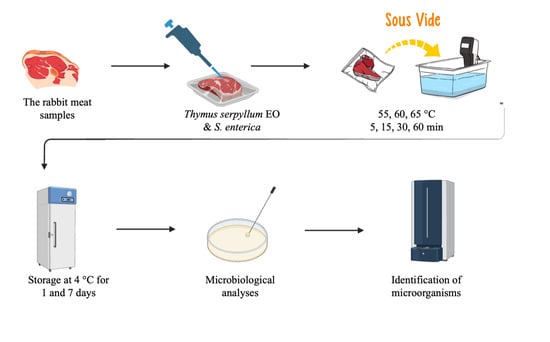
2. Materials and Methods
2.1. Preparation of Bacterial Suspension
2.2. Essential Oil
2.3. Sample of Rabbit Meat Preparation
2.4. Cultivation of Samples
2.5. Microorganisms Identification with Mass Spectrometry
2.6. Statistical Analyses
3. Results
3.1. Microbiological Analyses of Rabbit Sous Vide Meat at 1 and 7 Days
3.2. Identified Species of Bacteria at Days 1 and 7
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cullere, M.; Dalle Zotte, A. Rabbit Meat Production and Consumption: State of Knowledge and Future Perspectives. Meat Sci. 2018, 143, 137–146. [Google Scholar] [CrossRef]
- Dalle Zotte, A.; Szendrő, Z. The Role of Rabbit Meat as Functional Food. Meat Sci. 2011, 88, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Rasinska, E.; Czarniecka-Skubina, E.; Rutkowska, J. Fatty Acid and Lipid Contents Differentiation in Cuts of Rabbit Meat. CyTA-J. Food 2018, 16, 807–813. [Google Scholar] [CrossRef]
- Rodríguez-Calleja, J.M.; García-López, I.; García-López, M.-L.; Santos, J.A.; Otero, A. Rabbit Meat as a Source of Bacterial Foodborne Pathogens. J. Food Prot. 2006, 69, 1106–1112. [Google Scholar] [CrossRef]
- Bonardi, S. Isolation of Salmonella Enterica Serotype Brancaster from Slaughtered Rabbits. Available online: https://air.unipr.it/handle/11381/1448694 (accessed on 25 November 2023).
- Catellani, P.; Malocco, C.; Rasetti, M. Possibile ruolo dei conigli allevati come portatori asintomatici di Salmonella spp.-Indagini in fase di macellazione. (Possible role of breed rabbits as asymptomatic carriers of Salmonella spp. at slaughterhouse). Atti. Soc. Ital. Sci. Vet. LIII. 1999, 321–322. [Google Scholar]
- Hamed, A.M.; Eid, M.; El-Bakrey, R. A Review of Rabbit Diseases in Egypt. Ind. Bull. Ani Vet. Sci. 2014, 23, 185–194. [Google Scholar] [CrossRef]
- Jordan, E.; Egan, J.; Dullea, C.; Ward, J.; McGillicuddy, K.; Murray, G.; Murphy, A.; Bradshaw, B.; Leonard, N.; Rafter, P.; et al. Salmonella Surveillance in Raw and Cooked Meat and Meat Products in the Republic of Ireland from 2002 to 2004. Int. J. Food Microbiol. 2006, 112, 66–70. [Google Scholar] [CrossRef]
- Collard, J.M.; Bertrand, S.; Dierick, K.; Godard, C.; Wildemauwe, C.; Vermeersch, K.; Duculot, J.; Van Immerseel, F.; Pasmans, F.; Imberechts, H.; et al. Drastic Decrease of Salmonella Enteritidis Isolated from Humans in Belgium in 2005, Shift in Phage Types and Influence on Foodborne Outbreaks. Epidemiol. Infect. 2008, 136, 771–781. [Google Scholar] [CrossRef]
- Terentjeva, M.; Avsejenko, J.; Streikiša, M.; Utināne, A.; Kovaļenko, K.; Bērziņš, A. Prevalence and Antimicrobial Resistance of Salmonella in Meat and Meat Products in Latvia. Ann. Agric. Environ. Med. 2017, 24, 317–321. [Google Scholar] [CrossRef]
- Leistner, L.; Gorris, L.G.M. Food Preservation by Hurdle Technology. Trends Food Sci. Technol. 1995, 6, 41–46. [Google Scholar] [CrossRef]
- Baldwin, D. Sous Vide Cooking. In Handbook of Molecular Gastronomy, 1st ed.; Burke, R.M., Kelly, A.L., Lavelle, C., Kientza, H.T.V., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 531–535. ISBN 978-0-429-16870-3. [Google Scholar]
- Latoch, A.; Głuchowski, A.; Czarniecka-Skubina, E. Sous-Vide as an Alternative Method of Cooking to Improve the Quality of Meat: A Review. Foods 2023, 12, 3110. [Google Scholar] [CrossRef] [PubMed]
- Karyotis, D.; Skandamis, P.N.; Juneja, V.K. Thermal Inactivation of Listeria monocytogenes and Salmonella spp. in Sous-Vide Processed Marinated Chicken Breast. Food Res. Int. 2017, 100, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Küçükaydın, S.; Tel-Çayan, G.; Duru, M.E.; Kesdek, M.; Öztürk, M. Chemical Composition and Insecticidal Activities of the Essential Oils and Various Extracts of Two Thymus Species: Thymus cariensis and Thymus cilicicus. Toxin Rev. 2021, 40, 1461–1471. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Mousavi Khaneghah, A.; Gavahian, M.; Marszałek, K.; Eş, I.; Munekata, P.E.S.; Ferreira, I.C.F.R.; Barba, F.J. Understanding the Potential Benefits of Thyme and Its Derived Products for Food Industry and Consumer Health: From Extraction of Value-Added Compounds to the Evaluation of Bioaccessibility, Bioavailability, Anti-Inflammatory, and Antimicrobial Activities. Crit. Rev. Food Sci. Nutr. 2019, 59, 2879–2895. [Google Scholar] [CrossRef] [PubMed]
- Font-i-Furnols, M.; Guerrero, L. Consumer Preference, Behavior and Perception about Meat and Meat Products: An Overview. Meat Sci. 2014, 98, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Kiprotich, S.; Mendonça, A.; Dickson, J.; Shaw, A.; Thomas-Popo, E.; White, S.; Moutiq, R.; Ibrahim, S.A. Thyme Oil Enhances the Inactivation of Salmonella enterica on Raw Chicken Breast Meat During Marination in Lemon Juice with Added Yucca Schidigera Extract. Front. Nutr. 2021, 7, 619023. [Google Scholar] [CrossRef]
- Boskovic, M.; Djordjevic, J.; Ivanovic, J.; Janjic, J.; Zdravkovic, N.; Glisic, M.; Glamoclija, N.; Baltic, B.; Djordjevic, V.; Baltic, M. Inhibition of Salmonella by Thyme Essential Oil and Its Effect on Microbiological and Sensory Properties of Minced Pork Meat Packaged under Vacuum and Modified Atmosphere. Int. J. Food Microbiol. 2017, 258, 58–67. [Google Scholar] [CrossRef]
- Shin, W.-S.; Moon, G.-S.; Park, J.-H. Growth of Clostridium perfringens Spores Inoculated in Sous-Vide Processed Korean Traditional Galbijm under Different Storage Conditions. Food Sci. Biotechnol. 2014, 23, 505–509. [Google Scholar] [CrossRef]
- Nyati, H. Survival Characteristics and the Applicability of Predictive Mathematical Modelling to Listeria monocytogenes Growth in Sous Vide Products. Int. J. Food Microbiol. 2000, 56, 123–132. [Google Scholar] [CrossRef]
- Juneja, V.K.; Marks, H.M. Characterizing Asymptotic D-Values for Salmonella Spp. Subjected to Different Heating Rates in Sous-Vide Cooked Beef. Innov. Food Sci. Emerg. Technol. 2003, 4, 395–402. [Google Scholar] [CrossRef]
- Duan, Z.; Hansen, T.H.; Hansen, T.B.; Dalgaard, P.; Knøchel, S. Predicting Outgrowth and Inactivation of Clostridium perfringens in Meat Products during Low Temperature Long Time Heat Treatment. Int. J. Food Microbiol. 2016, 230, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Terentjeva, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.Ł.; Kačániová, M. Thymus serpyllum Essential Oil and Its Biological Activity as a Modern Food Preserver. Plants 2021, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- Gál, R.; Čmiková, N.; Kačániová, M.; Mokrejš, P. Sage Essential Oil as an Antimicrobial Agent against Salmonella enterica during Beef Sous Vide Storage. Foods 2023, 12, 4172. [Google Scholar] [CrossRef]
- Gál, R.; Čmiková, N.; Prokopová, A.; Kačániová, M. Antilisterial and Antimicrobial Effect of Salvia officinalis Essential Oil in Beef Sous-Vide Meat during Storage. Foods 2023, 12, 2201. [Google Scholar] [CrossRef] [PubMed]
- Dalle Zotte, A. Perception of Rabbit Meat Quality and Major Factors Influencing the Rabbit Carcass and Meat Quality. Livest. Prod. Sci. 2002, 75, 11–32. [Google Scholar] [CrossRef]
- Schmidt, R.H.; Rodrick, G.E. Food Safety Handbook, 1st ed.; Wiley: Hoboken, NJ, USA, 2003; ISBN 978-0-471-21064-1. [Google Scholar]
- Hulot, F.; Ouhayoun, J. Muscular pH And Related Traits In Rabbits: A Review. World Rabbit Sci. 2010, 7, 15–36. [Google Scholar] [CrossRef]
- Khalafalla, F.A. Microbiological Status of Rabbit Carcases in Egypt. Z. Lebensm. Unters. Forch 1993, 196, 233–235. [Google Scholar] [CrossRef]
- Soletska, A.; Krasota, A. Prospects Of Applying Vacuum Technology In The Manufacture Of Culinary Poultry Meat Products. Food Environ. Saf. J. 2017, 15, 3–9. [Google Scholar]
- Hong, G.-E.; Kim, J.-H.; Ahn, S.-J.; Lee, C.-H. Changes in Meat Quality Characteristics of the Sous-Vide Cooked Chicken Breast during Refrigerated Storage. Korean J. Food Sci. Anim. Resour. 2015, 35, 757–764. [Google Scholar] [CrossRef]
- Bıyıklı, M.; Akoğlu, A.; Kurhan, Ş.; Akoğlu, İ.T. Effect of Different Sous Vide Cooking Temperature-Time Combinations on the Physicochemical, Microbiological, and Sensory Properties of Turkey Cutlet. Int. J. Gastr Food Sci. 2020, 20, 100204. [Google Scholar] [CrossRef]
- Demirci, A.; Ngadi, M.O.; Neetoo, H. Microbial Decontamination in the Food Industry: Novel Methods and Applications; Woodhead publishing series in food science and technology; Woodhead Publishing: Oxford, UK, 2012; ISBN 978-0-85709-085-0. [Google Scholar]
- Akoğlu, I.; Bıyıklı, M.; Akoğlu, A.; Kurhan, Ş. Determination of the Quality and Shelf Life of Sous Vide Cooked Turkey Cutlet Stored at 4 and 12 °C. Rev. Bras. Cienc. Avic. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Juneja, V.K.; Eblen, B.S. Heat Inactivation of Salmonella typhimurium DT104 in Beef as Affected by Fat Content. Lett. Appl. Microbiol. 2000, 30, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Maurer, J.L.; Orta-Ramirez, A.; Ryser, E.T.; Smith, D.M. Thermal Inactivation of Salmonella spp., Salmonella typhimurium DT104, and Escherichia coli 0157:H7 in Ground Beef. J. Food Sci. 2001, 66, 1164–1168. [Google Scholar] [CrossRef]
- Vasan, A.; Ingham, S.C.; Ingham, B.H. Comparative Effect of Heat Shock on Survival of O157:H7 and Non-O157 Shiga Toxigenic Escherichia coli and Salmonella in Lean Beef with or without Moisture-Enhancing Ingredients. J. Food Prot. 2017, 80, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Sarjit, A.; Ravensdale, J.T.; Coorey, R.; Fegan, N.; Dykes, G.A. Survival of Salmonella on Red Meat in Response to Dry Heat. J. Food Prot. 2021, 84, 372–380. [Google Scholar] [CrossRef] [PubMed]
- De Jong, A.E.I.; Van Asselt, E.D.; Zwietering, M.H.; Nauta, M.J.; De Jonge, R. Extreme Heat Resistance of Food Borne Pathogens Campylobacter jejuni, Escherichia coli, and Salmonella typhimurium on Chicken Breast Fillet during Cooking. Int. J. Microbiol. 2012, 2012, 196841. [Google Scholar] [CrossRef]
- McCann, M.S.; McGovern, A.C.; McDowell, D.A.; Blair, I.S.; Sheridan, J.J. Surface Decontamination of Beef Inoculated with Salmonella typhimurium DT104 or Escherichia coli O157:H7 Using Dry Air in a Novel Heat Treatment Apparatus. J. Appl. Microbiol. 2006, 101, 1177–1187. [Google Scholar] [CrossRef]
- Jarvis, N.A.; O’Bryan, C.A.; Dawoud, T.M.; Park, S.H.; Kwon, Y.M.; Crandall, P.G.; Ricke, S.C. An Overview of Salmonella Thermal Destruction during Food Processing and Preparation. Food Control 2016, 68, 280–290. [Google Scholar] [CrossRef]
- Orta-Ramirez, A.; Marks, B.P.; Warsow, C.R.; Booren, A.M.; Ryser, E.T. Enhanced Thermal Resistance of Salmonella in Whole Muscle Compared to Ground Beef. J. Food Sci. 2005, 70, m359–m362. [Google Scholar] [CrossRef]
- Azrad, M.; Keness, Y.; Nitzan, O.; Pastukh, N.; Tkhawkho, L.; Freidus, V.; Peretz, A. Cheap and Rapid In-House Method for Direct Identification of Positive Blood Cultures by MALDI-TOF MS Technology. BMC Infect. Dis. 2019, 19, 72. [Google Scholar] [CrossRef]
- Koné, A.P.; Desjardins, Y.; Gosselin, A.; Cinq-Mars, D.; Guay, F.; Saucier, L. Plant Extracts and Essential Oil Product as Feed Additives to Control Rabbit Meat Microbial Quality. Meat Sci. 2019, 150, 111–121. [Google Scholar] [CrossRef]
- Śmiecińska, K.; Gugołek, A.; Kowalska, D. Effects of Garlic (Allium sativum L.) and Ramsons (Allium ursinum L.) on Lipid Oxidation and the Microbiological Quality, Physicochemical Properties and Sensory Attributes of Rabbit Meat Burgers. Animals 2022, 12, 1905. [Google Scholar] [CrossRef]
- El Abed, N.; Kaabi, B.; Smaali, M.I.; Chabbouh, M.; Habibi, K.; Mejri, M.; Marzouki, M.N.; Ben Hadj Ahmed, S. Chemical Composition, Antioxidant and Antimicrobial Activities of Thymus capitata Essential Oil with Its Preservative Effect against Listeria monocytogenes Inoculated in Minced Beef Meat. Evid.-Based Complement. Altern. Med. 2014, 2014, 152487. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naime, W.A.; Fahim, J.R.; Fouad, M.A.; Kamel, M.S. Antibacterial, Antifungal, and GC–MS Studies of Melissa officinalis. S. Afr. J. Bot. 2019, 124, 228–234. [Google Scholar] [CrossRef]
- Jayari, A.; El Abed, N.; Jouini, A.; Mohammed Saed Abdul-Wahab, O.; Maaroufi, A.; Ben Hadj Ahmed, S. Antibacterial Activity of Thymus capitatus and Thymus algeriensis Essential Oils against Four Food-borne Pathogens Inoculated in Minced Beef Meat. J. Food Saf. 2018, 38, e12409. [Google Scholar] [CrossRef]
- Pesavento, G.; Calonico, C.; Bilia, A.R.; Barnabei, M.; Calesini, F.; Addona, R.; Mencarelli, L.; Carmagnini, L.; Di Martino, M.C.; Lo Nostro, A. Antibacterial Activity of Oregano, Rosmarinus and Thymus Essential Oils against Staphylococcus aureus and Listeria monocytogenes in Beef Meatballs. Food Control 2015, 54, 188–199. [Google Scholar] [CrossRef]
- Barbosa, L.N.; Rall, V.L.M.; Fernandes, A.A.H.; Ushimaru, P.I.; Da Silva Probst, I.; Fernandes, A. Essential Oils Against Foodborne Pathogens and Spoilage Bacteria in Minced Meat. Foodborne Pathog. Dis. 2009, 6, 725–728. [Google Scholar] [CrossRef]
- Amariei, S.; Poroch-Seriţan, M.; Gutt, G.; Oroian, M.; Ciornei, E. Rosemary, Thyme and Oregano Essential Oils Influence on Physicochemical Properties and Microbiological Stability of Minced Meat. J. Microbiol. Biotechnol. Food Sci. 2016, 6, 670–676. [Google Scholar] [CrossRef]
- Solomakos, N.; Govaris, A.; Koidis, P.; Botsoglou, N. The Antimicrobial Effect of Thyme Essential Oil, Nisin and Their Combination against Escherichia coli O157:H7 in Minced Beef during Refrigerated Storage. Meat Sci. 2008, 80, 159–166. [Google Scholar] [CrossRef]
- Gouveia, A.R.; Alves, M.; Silva, J.A.; Saraiva, C. The Antimicrobial Effect of Rosemary and Thyme Essential Oils Against Listeria monocytogenes in Sous Vide Cook-Chill Beef During Storage. Procedia Food Sci. 2016, 7, 173–176. [Google Scholar] [CrossRef]
- Chien, S.-Y.; Sheen, S.; Sommers, C.H.; Sheen, L.-Y. Modeling the Inactivation of Intestinal Pathogenic Escherichia coli O157:H7 and Uropathogenic E. coli in Ground Chicken by High Pressure Processing and Thymol. Front. Microbiol. 2016, 7, 920. [Google Scholar] [CrossRef]
- Radünz, M.; Dos Santos Hackbart, H.C.; Camargo, T.M.; Nunes, C.F.P.; De Barros, F.A.P.; Dal Magro, J.; Filho, P.J.S.; Gandra, E.A.; Radünz, A.L.; Da Rosa Zavareze, E. Antimicrobial Potential of Spray Drying Encapsulated Thyme (Thymus vulgaris) Essential Oil on the Conservation of Hamburger-like Meat Products. Int. J. Food Microbiol. 2020, 330, 108696. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kačániová, M.; Čmiková, N.; Kluz, M.I.; Akacha, B.B.; Saad, R.B.; Mnif, W.; Waszkiewicz-Robak, B.; Garzoli, S.; Hsouna, A.B. Anti-Salmonella Activity of Thymus serpyllum Essential Oil in Sous Vide Cook–Chill Rabbit Meat. Foods 2024, 13, 200. https://doi.org/10.3390/foods13020200
Kačániová M, Čmiková N, Kluz MI, Akacha BB, Saad RB, Mnif W, Waszkiewicz-Robak B, Garzoli S, Hsouna AB. Anti-Salmonella Activity of Thymus serpyllum Essential Oil in Sous Vide Cook–Chill Rabbit Meat. Foods. 2024; 13(2):200. https://doi.org/10.3390/foods13020200
Chicago/Turabian StyleKačániová, Miroslava, Natália Čmiková, Maciej Ireneusz Kluz, Boutheina Ben Akacha, Rania Ben Saad, Wissem Mnif, Bożena Waszkiewicz-Robak, Stefania Garzoli, and Anis Ben Hsouna. 2024. "Anti-Salmonella Activity of Thymus serpyllum Essential Oil in Sous Vide Cook–Chill Rabbit Meat" Foods 13, no. 2: 200. https://doi.org/10.3390/foods13020200