How Biological Activity in Sea Cucumbers Changes as a Function of Species and Tissue
Abstract
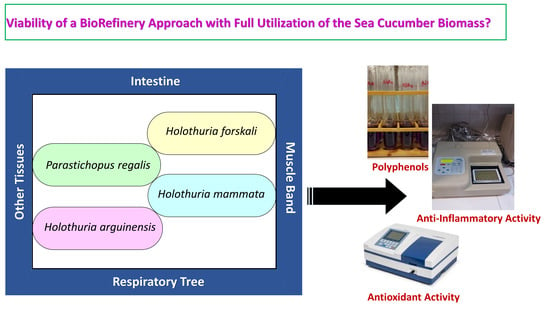
:1. Introduction
2. Materials and Methods
2.1. Sample Source, Collection, and Preparation
2.2. Preparation of Extracts
2.3. Total Polyphenol Content
2.4. Antioxidant Activity as Measured Using the DPPH Method
- A0—Absorbance of the blank; and
- Asample—Absorbance of the sample.
2.5. Antioxidant Activity as Measured Using the FRAP Method
2.6. Anti-Inflammatory Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Polyphenols and Antioxidant Activity
3.2. Anti-Inflammatory Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warnau, M.; Dutrieux, S.; Ledent, G.; Rodriguez y Baena, A.M.; Dubois, P. Heavy metals in the sea cucumber Holothuria tubulosa (Echinodermata) from the Mediterranean Posidonia oceanica ecosystem: Body compartment, seasonal, geographical and batymetric variations. Environ. Bioindic. 2006, 1, 268–285. [Google Scholar] [CrossRef]
- Birch, G.F. Determination of sediment metal background concentrations and enrichment in marine environments—A critical review. Sci. Total Environ. 2017, 580, 813–831. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed]
- Lobine, D.; Rengasamy, K.R.R.; Mahomoodally, M.F. Functional foods and bioactive ingredients harnessed from the ocean: Current status and future perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 5794–5823. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.W.; Conand, C.; Uthicke, S.; Byrne, M. Ecological roles of exploited sea cucumbers. In Oceanography and Marine Biology: An Annual Review; Hughes, R.N., Hughes, D.J., Smith, I.P., Dale, A.C., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2016; pp. 367–386. [Google Scholar]
- Khotimchenko, Y.S. The nutritional value of holothurians. Rus. J. Mar. Biol. 2015, 41, 409–423. [Google Scholar] [CrossRef]
- Khotimchenko, Y.S. Pharmacological potential of sea cucumbers. Int. J. Mol. Sci. 2018, 19, 1342. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Gao, Z.; Zhang, L.; Guo, F.; Chen, Y.; Li, Y.; Huang, C. Saponin-enriched sea cucumber extracts exhibit an antiobesity effect through inhibition of pancreatic lipase activity and upregulation of LXR-β signaling. Pharm. Biol. 2016, 54, 1312–1325. [Google Scholar] [CrossRef] [PubMed]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Wu, F.-J.; Xue, Y.; Liu, X.-F.; Xue, C.-H.; Wang, J.-F.; Du, L.; Takahashi, K.; Wang, Y.-M. The protective effect of eicosapentaenoic acid-enriched phospholipids from sea cucumber Cucumaria frondosa on oxidative stress in PC12 cells and SAMP8 mice. Neurochem. Int. 2014, 64, 9–17. [Google Scholar] [CrossRef]
- Pangestuti, R.; Arifin, Z. Medicinal and health benefit effects of functional sea cucumbers. J. Trad. Compl. Med. 2018, 8, 341–351. [Google Scholar] [CrossRef]
- Santos, R.; Dias, S.; Pinteus, S.; Silva, J.; Alves, C.; Tecelão, C.; Pedrosa, R.; Pombo, A. Sea cucumber Holothuria forskali, a new resource for aquaculture? Reproductive biology and nutraceutical approach. Aquac. Res. 2016, 47, 2307–2323. [Google Scholar] [CrossRef]
- Venâncio, E.; Félix, P.M.; Brito, A.C.; Sousa, J.; Azevedo e Silva, F.; Simões, T.; Narciso, L.; Amorim, A.; Dâmaso, L.; Pombo, A. Do broodstock diets influence viability and larval development of Holothuria mammata? Aquaculture 2021, 536, 736431. [Google Scholar] [CrossRef]
- Domínguez-Godino, J.A.; González-Wangüemert, M. Holothuria arguinensis: A new sea cucumber species for aquaculture. SPC Beche-De-Mer Inf. Bull. 2019, 39, 60–64. [Google Scholar]
- Madruga, A.S.; Félix, P.M.; Sousa, J.; Azevedo e Silva, F.; Brito, A.C.; Mendes, S.; Pombo, A. Effect of rearing temperature in the growth of hatchery reared juveniles of the sea cucumber Holothuria arguinensis (Koehler & Vaney, 1906). Aquaculture 2023, 562, 738809. [Google Scholar]
- Carletti, A.; Cardoso, C.; Lobo-Arteaga, J.; Sales, S.; Julião, D.; Ferreira, I.; Chainho, P.; Dionísio, M.A.; Gaudêncio, M.J.; Afonso, C.; et al. Antioxidant and anti-inflammatory extracts from sea cucumbers and tunicates induce a pro-osteogenic effect in zebrafish larvae. Front. Nutr. 2022, 9, 888360. [Google Scholar] [CrossRef] [PubMed]
- García, J.; Méndez, D.; Álvarez, M.; Sanmartin, B.; Vazquez Sobrado, R.; Regueiro, L.; Atanassova, M. Design of novel functional food products enriched with bioactive extracts from holothurians for meeting the nutritional needs of the elderly. LWT Food Sci. Technol. 2019, 109, 55–62. [Google Scholar] [CrossRef]
- Roggatz, C.C.; González-Wangüemert, M.; Pereira, H.; Vizetto-Duarte, C.; Rodrigues, M.J.; Barreira, L.; da Silva, M.M.; Varela, J.; Custódio, L. A first glance into the nutritional properties of the sea cucumber Parastichopus regalis from the Mediterranean Sea (SE Spain). Nat. Prod. Res. 2018, 32, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.-Y.; Choi, B.-D.; Moon, S.-K.; Lee, J.-S.; Jeong, W.-G. Fatty acid composition of 35 species of marine invertebrates. J. Fish. Sci. Technol. 1998, 1, 232–241. [Google Scholar]
- Telahigue, K.; Ghali, R.; Nouiri, E.; Labidi, A.; Hajji, T. Antibacterial activities and bioactive compounds of the ethyl acetate extract of the sea cucumber Holothuria forskali from Tunisian coasts. J. Mar. Biol. Assoc. U. K. 2020, 100, 229–237. [Google Scholar] [CrossRef]
- Ramón, M.; Lleonart, J.; Massutí, E. Royal cucumber (Stichopus regalis) in the northwestern Mediterranean: Distribution pattern and fishery. Fish. Res. 2010, 105, 21–27. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; Van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Dave, D.; Shahidi, F. Antioxidant potential of sea cucumbers and their beneficial effects on human health. Mar. Drugs 2022, 20, 521. [Google Scholar] [CrossRef] [PubMed]
- Roggatz, C.C.; González-Wangüemert, M.; Pereira, H.; Rodrigues, M.J.; da Silva, M.M.; Barreira, L.; Varela, J.; Custódio, L. First report of the nutritional profile and antioxidant potential of Holothuria arguinensis, a new resource for aquaculture in Europe. Nat. Prod. Res. 2016, 30, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Pranweerapaiboon, K.; Apisawetakan, S.; Nobsathian, S.; Itharat, A.; Sobhon, P.; Chaithirayanon, K. An ethyl-acetate fraction of Holothuria scabra modulates inflammation in vitro through inhibiting the production of nitric oxide and pro-inflammatory cytokines via NF-κB and JNK pathways. Inflammopharmacology 2020, 28, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Yeo, J.D.; Dave, D.; Shahidi, F. Phenolic compounds and antioxidant capacity of sea cucumber (Cucumaria frondosa) processing discards as affected by High-Pressure Processing (HPP). Antioxidants 2022, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Mamelona, J.; Pelletier, É.; Girard-Lalancette, K.; Legault, J.; Karboune, S.; Kermasha, S. Quantification of phenolic contents and antioxidant capacity of Atlantic sea cucumber, Cucumaria frondosa. Food Chem. 2007, 104, 1040–1047. [Google Scholar] [CrossRef]
- Dakrory, A.I.; Fahmy, S.R.; Soliman, A.M.; Mohamed, A.S.; Amer, S.A.M. Protective and curative effects of the sea cucumber Holothuria atra extract against DMBA-induced hepatorenal diseases in rats. BioMed Res. Int. 2015, 2015, 563652. [Google Scholar] [CrossRef]
- Hawa, I.; Zulaikah, M.; Jamaludin, M.; Zainal Abidin, A.A.; Kaswandi, M.A.; Ridzwan, B.H. The potential of the coelomic fluid of sea cucumber as an antioxidant. Malays. J. Nutr. 1999, 5, 55–59. [Google Scholar]
- Zmemlia, N.; Bejaoui, S.; Khemiri, I.; Bouriga, N.; Louiz, I.; El-Bok, S.; Ben-Attia, M.; Souli, A. Biochemical composition and antioxidant potential of the edible Mediterranean sea cucumber Holothuria tubulosa. Grasas Aceites 2020, 71, e364. [Google Scholar] [CrossRef]
- Dimitrios, B. Sources of natural phenolic antioxidants. Trends Food Sci. Technol. 2006, 17, 505–512. [Google Scholar] [CrossRef]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef] [PubMed]
- Esmat, A.Y.; Said, M.M.; Soliman, A.A.; El-Masry, K.S.; Badiea, E.A. Bioactive compounds, antioxidant potential, and hepatoprotective activity of sea cucumber (Holothuria atra) against thioacetamide intoxication in rats. Nutrition 2013, 29, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Senadheera, T.R.; Dave, D.; Shahidi, F. Phenolic profiles of Atlantic sea cucumber (Cucumaria frondosa) tentacles and their biological properties. Food Res. Int. 2023, 163, 112262. [Google Scholar] [CrossRef]
- Wulandari, D.A.; Gustini, N.; Murniasih, T.; Bayu, A.; Sari, M.; Syahputra, G.; Harahap, I.A.; Rasyid, A.; Moria, S.B.; Rahmawati, S.I.; et al. Nutritional value and biological activities of sea cucumber Holothuria scabra cultured in the open pond system. J. Aquat. Food Prod. Technol. 2022, 31, 599–614. [Google Scholar] [CrossRef]
- Kareh, M.; El Nahas, R.; Al-Aaraj, L.; Al-Ghadban, S.; Al Deen, N.N.; Saliba, N.; El-Sabban, M.; Talhouk, R. Anti-proliferative and anti-inflammatory activities of the sea cucumber Holothuria polii aqueous extract. SAGE Open Med. 2018, 6, 2050312118809541. [Google Scholar] [CrossRef]
- Moradi, Y.; Nazemi, M.; Safari, R. Evaluation of anti-inflammatory effects of methanolic extract of Persian Gulf sea cucumber (Holothuria leucospilota) on rats. J. Fish. (Iran. J. Nat. Resour.) 2020, 73, 91–100. [Google Scholar]
- Andriawan, S.; Hermawan, D.; Maidah, E.N.; Cahyani, D.; Sanoesi, E. Anti-inflammatory effects of Holothuria scabra extract on Pangasianodon hypophthalmus tissues infected with Aeromonas hydrophila. AACL Bioflux 2021, 14, 1259–1270. [Google Scholar]
- Yamazaki, Y.; Sakai, Y.; Yu, J.; Mino, S.; Sawabe, T. Tracking the dynamics of individual gut microbiome of sea cucumber Apostichopus japonicus during gut regeneration. PeerJ 2020, 8, e10260. [Google Scholar] [CrossRef]
- Passos, F.R.S.; Araújo-Filho, H.G.; Monteiro, B.S.; Shanmugam, S.; Araújo, A.A.S.; Almeida, J.R.G.S.; Thangaraj, P.; Quintans Júnior, L.J.; Quintans, J.S.S. Anti-inflammatory and modulatory effects of steroidal saponins and sapogenins on cytokines: A review of pre-clinical research. Phytomedicine 2022, 96, 153842. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Himaya, S.W. Triterpene glycosides from sea cucumbers and their biological activities. Adv. Food Nutr. Res. 2012, 65, 297–319. [Google Scholar] [PubMed]
- Mitu, S.A.; Bose, U.; Suwansa-Ard, S.; Turner, L.H.; Zhao, M.; Elizur, A.; Ogbourne, S.M.; Shaw, P.N.; Cummins, S.F. Evidence for a saponin biosynthesis pathway in the body wall of the commercially significant sea cucumber Holothuria scabra. Mar. Drugs 2017, 15, 349. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, Y.; Zhang, W.; Chataway, T.; Franco, C. Structural elucidation of novel saponins in the sea cucumber Holothuria lessoni. Mar. Drugs 2014, 12, 4439–4473. [Google Scholar] [CrossRef]
- Van Dyck, S.; Caulier, G.; Todesco, M.; Gerbaux, P.; Fournier, I.; Wisztorski, M.; Flammang, P. The triterpene glycosides of Holothuria forskali: Usefulness and efficiency as a chemical defense mechanism against predatory fish. J. Exp. Biol. 2011, 214, 1347–1356. [Google Scholar] [CrossRef]

| Tissue | Total Polyphenol (mg GAE/100 g dw) | |||
|---|---|---|---|---|
| Parastichopus regalis | Holothuria mammata | Holothuria forskali | Holothuria arguinensis | |
| Intestine | 18.8 ± 0.0 bA | 15.9 ± 0.5 cA | 9.7 ± 0.8 dA | 37.5 ± 0.3 aB |
| Muscle band | 29.8 ± 0.6 aB | 56.0 ± 1.4 cD | 53.1 ± 0.9 bD | 62.5 ± 0.8 dD |
| Respiratory tree | 21.0 ± 0.8 aA | 49.0 ± 0.5 bC | 48.5 ± 0.3 bC | 76.4 ± 1.2 cE |
| Body Wall | 21.6 ± 0.6 aA | 32.4 ± 0.6 cB | 37.3 ± 0.5 dB | 28.0 ± 0.3 bA |
| Gonads | 15.4 ± 0.8 aA | 60.4 ± 0.5 cE | NA | 49.9 ± 0.5 bC |
| Tissue | DPPH (mg AAE/100 g dw) | |||
|---|---|---|---|---|
| Parastichopus regalis | Holothuria mammata | Holothuria forskali | Holothuria arguinensis | |
| Intestine | 1.9 ± 0.5 aA | 8.5 ± 0.7 bB | 8.4 ± 1.5 bB | 15.1 ± 0.8 cC |
| Muscle band | 5.6 ± 0.1 aB | 11.3 ± 0.9 bC | 13.9 ± 0.6 cC | 9.4 ± 0.1 bB |
| Respiratory tree | ND aA | 11.9 ± 0.8 dC | 3.3 ± 1.2 bA | 8.4 ± 0.6 cB |
| Body Wall | 4.7 ± 0.3 aB | 4.7 ± 1.0 aA | 6.5 ± 1.0 aB | 4.1 ± 1.0 aA |
| Gonads | 3.5 ± 0.2 aB | 2.6 ± 0.9 aA | NA | 13.6 ± 0.7 bC |
| Tissue | FRAP (μmol Fe2+/g dw) | |||
|---|---|---|---|---|
| Parastichopus regalis | Holothuria mammata | Holothuria forskali | Holothuria arguinensis | |
| Intestine | 6.4 ± 0.1 aB | 22.5 ± 0.3 bC | 21.7 ± 0.2 bC | 28.5 ± 0.6 cE |
| Muscle band | 23.5 ± 0.3 aD | 25.1 ± 0.4 bE | 28.4 ± 0.4 cD | 24.5 ± 0.0 abC |
| Respiratory tree | 5.4 ± 0.2 aA | 23.8 ± 0.4 dD | 12.9 ± 0.4 bA | 20.9 ± 0.3 cB |
| Body Wall | 15.2 ± 0.1 aC | 14.8 ± 0.4 aB | 20.0 ± 0.1 bB | 15.3 ± 0.4 aA |
| Gonads | 15.9 ± 0.1 bC | 8.0 ± 0.3 aA | NA | 27.1 ± 0.3 cD |
| Tissue | Anti-Inflammatory Activity (% COX-2 Inhibition) | |||
|---|---|---|---|---|
| Parastichopus regalis | Holothuria mammata | Holothuria forskali | Holothuria arguinensis | |
| Intestine | 29.8 ± 1.5 aB | 31.6 ± 5.8 aB | 13.1 ± 4.4 bA | 15.5 ± 6.0 bA |
| Muscle band | 2.6 ± 0.0 bA | 13.1 ± 16.3 bA | 67.2 ± 2.5 cB | ND aB |
| Respiratory tree | 58.4 ± 4.6 bC | 76.3 ± 6.3 aC | 59.5 ± 3.6 bB | 20.1 ± 12.9 cA |
| Body Wall | 12.7 ± 1.6 aA | 2.3 ± 0.0 aA | 7.8 ± 0.0 aA | 6.8 ± 2.3 aA |
| Gonads | 94.6 ± 4.0 aD | 10.2 ± 6.4 bA | NA | ND cB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sales, S.; Lourenço, H.M.; Bandarra, N.M.; Afonso, C.; Matos, J.; Botelho, M.J.; Pessoa, M.F.; Félix, P.M.; Veronez, A.; Cardoso, C. How Biological Activity in Sea Cucumbers Changes as a Function of Species and Tissue. Foods 2024, 13, 35. https://doi.org/10.3390/foods13010035
Sales S, Lourenço HM, Bandarra NM, Afonso C, Matos J, Botelho MJ, Pessoa MF, Félix PM, Veronez A, Cardoso C. How Biological Activity in Sea Cucumbers Changes as a Function of Species and Tissue. Foods. 2024; 13(1):35. https://doi.org/10.3390/foods13010035
Chicago/Turabian StyleSales, Sabrina, Helena M. Lourenço, Narcisa M. Bandarra, Cláudia Afonso, Joana Matos, Maria João Botelho, Maria Fernanda Pessoa, Pedro M. Félix, Arthur Veronez, and Carlos Cardoso. 2024. "How Biological Activity in Sea Cucumbers Changes as a Function of Species and Tissue" Foods 13, no. 1: 35. https://doi.org/10.3390/foods13010035