A Suitable HPLC-MS/MS Methodology for the Detection of Oxytetracycline, Enrofloxacin, and Sulfachloropyridazine Residues in Lettuce Plants
Abstract
:1. Introduction
2. Materials and Methods
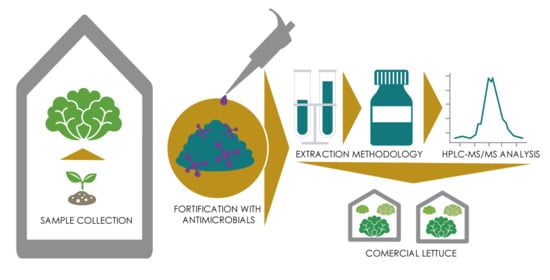
2.1. Sample Collection
2.2. Standards, Reagents, and Chemicals
2.3. Pre-Treatment and Extraction Proceedure of Antimicrobial Residues from Lettuce Samples
2.4. Instrumental Analysis
2.5. In-House Validation of the Analytical Method
2.6. Detection of Antimicrobial Drug Residues in Commercially Available Lettuce
3. Results
3.1. Implementation and Optimization of the Analytical Methodology
3.2. In-House Validation of the Analytical Methodology on Lettuce Samples
3.2.1. Specificity
3.2.2. Detection Range
3.2.3. Linearity of Calibration Curves
3.2.4. Recovery and Precision
3.3. Analysis of Antimicrobial Concentrations in Commercial Lettuce
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geng, J.; Liu, X.; Wang, J.; Li, S. Accumulation and Risk Assessment of Antibiotics in Edible Plants Grown in Contaminated Farmlands: A Review. Sci. Total Environ. 2022, 853, 158616. [Google Scholar] [CrossRef] [PubMed]
- Serwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Mora-Gamboa, M.P.C.; Rincón-Gamboa, S.M.; Ardila-Leal, L.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Quevedo-Hidalgo, B.E. Impact of Antibiotics as Waste, Physical, Chemical, and Enzymatical Degradation: Use of Laccases. Molecules 2022, 27, 4436. [Google Scholar] [CrossRef]
- Rocha, D.C.; Da Silva Rocha, C.; Tavares, D.S.; De Morais Calado, S.L.; Gomes, M.P. Veterinary Antibiotics and Plant Physiology: An Overview. Sci. Total Environ. 2021, 767, 144902. [Google Scholar] [CrossRef] [PubMed]
- Minden, V.; Deloy, A.; Volkert, A.M.; Leonhardt, S.D.; Pufal, G. Antibiotics Impact Plant Traits, Even at Small Concentrations. AoB Plants 2017, 9, plx010. [Google Scholar] [CrossRef] [PubMed]
- Carballo, M.; Rodríguez, A.; de la Torre, A. Phytotoxic Effects of Antibiotics on Terrestrial Crop Plants and Wild Plants: A Systematic Review. Arch. Environ. Contam. Toxicol. 2022, 82, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Arsène, M.M.J.; Davares, A.K.L.; Viktorovna, P.I.; Andreevna, S.L.; Sarra, S.; Khelifi, I.; Sergueïevna, D.M. The Public Health Issue of Antibiotic Residues in Food and Feed: Causes, Consequences, and Potential Solutions. Vet. World 2022, 15, 662–671. [Google Scholar] [CrossRef]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human Health Risk Assessment of Antibiotic Resistance Associated with Antibiotic Residues in the Environment: A Review. Environ. Res 2019, 169, 483–493. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef]
- Walsh, T.R.; Gales, A.C.; Laxminarayan, R.; Dodd, P.C. Antimicrobial Resistance: Addressing a Global Threat to Humanity. PLoS Med. 2023, 20, e1004264. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. FAO. Producción/Rendimiento de Lechuga y achicoria en Mundo + (Total) 1994–2021. Available online: https://www.fao.org/faostat/es/#data/QCL/visualize (accessed on 4 October 2023).
- Albero, B.; Tadeo, J.L.; Delgado, M.; del, M.; Miguel, E.; Pérez, R.A. Analysis of Multiclass Antibiotics in Lettuce by Liquid Chromatography–Tandem Mass Spectrometry to Monitor Their Plant Uptake. Molecules 2019, 24, 4066. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.M.; Rajapaksha, A.U.; Lim, J.E.; Vu, N.T.; Kim, I.S.; Kang, H.M.; Lee, S.S.; Ok, Y.S. Distribution and Accumulative Pattern of Tetracyclines and Sulfonamides in Edible Vegetables of Cucumber, Tomato, and Lettuce. J. Agric. Food Chem. 2015, 63, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Sallach, J.B.; Bartelt-Hunt, S.L.; Snow, D.D.; Li, X.; Hodges, L. Uptake of Antibiotics and Their Toxicity to Lettuce Following Routine Irrigation with Contaminated Water in Different Soil Types. Environ. Eng. Sci. 2018, 35, 887–896. [Google Scholar] [CrossRef]
- Sidhu, H.; O’Connor, G.; Kruse, J. Plant Toxicity and Accumulation of Biosolids-Borne Ciprofloxacin and Azithromycin. Sci. Total Environ. 2019, 648, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, G.; Chuang, Y.-H.; Hammerschmidt, R.; Zhang, W.; Boyd, S.A.; Li, H. Uptake of Cephalexin by Lettuce, Celery, and Radish from Water. Chemosphere 2021, 263, 127916. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Casas, M.E.; Mansilla, S.; Tadić, Đ.; Cañameras, N.; Carazo, N.; Portugal, J.; Piña, B.; Díez, S.; Bayona, J.M. Occurrence of Antibiotics in Lettuce (Lactuca Sativa L.) and Radish (Raphanus Sativus L.) Following Organic Soil Fertilisation under Plot-Scale Conditions: Crop and Human Health Implications. J. Hazard. Mater. 2022, 436, 129044. [Google Scholar] [CrossRef] [PubMed]
- Azanu, D.; Mortey, C.; Darko, G.; Weisser, J.J.; Styrishave, B.; Abaidoo, R.C. Uptake of Antibiotics from Irrigation Water by Plants. Chemosphere 2016, 157, 107–114. [Google Scholar] [CrossRef]
- Yu, X.; Liu, H.; Pu, C.; Chen, J.; Sun, Y.; Hu, L. Determination of Multiple Antibiotics in Leafy Vegetables Using QuEChERS-UHPLC-MS/MS. J. Sep. Sci. 2018, 41, 713–722. [Google Scholar] [CrossRef]
- World Organization for Animal Health. WOAH. 6to Annual Report on Antimicrobial Agents Intended for Use in Animals; OIE: París, Francia, 2022; p. 135. [Google Scholar]
- Servicio Agrícola y Ganadero. SAG. Sistema en Línea de Búsqueda de Medicamentos Veterinarios Autorizados Por el SAG. Available online: https://medicamentos.sag.gob.cl/ConsultaUsrPublico/BusquedaMedicamentos_1.asp (accessed on 2 September 2023).
- Peris-Vicente, J.; Peris-García, E.; Albiol-Chiva, J.; Durgbanshi, A.; Ochoa-Aranda, E.; Carda-Broch, S.; Bose, D.; Esteve-Romero, J. Liquid Chromatography, a Valuable Tool in the Determination of Antibiotics in Biological, Food and Environmental Samples. Microchem. J. 2022, 177, 107309. [Google Scholar] [CrossRef]
- Slana, M.; Pahor, V.; Cvitkovič Maričič, L.; Sollner-Dolenc, M. Excretion Pattern of Enrofloxacin after Oral Treatment of Chicken Broilers. J. Vet. Pharmacol. Therap. 2014, 37, 611–614. [Google Scholar] [CrossRef]
- Sversut, R.A.; Andrade Da Silva, A.; Mazon Cardoso, T.F.; Kassab, N.M.; Serrou Do Amaral, M.; Nunes Salgado, H.R. A Critical Review of Properties and Analytical Methods for the Determination of Oxytetracyline in Biological and Pharmaceutical Matrices. Crit. Rev. Anal. Chem. 2017, 47, 154–171. [Google Scholar] [CrossRef] [PubMed]
- Zinner, S.H.; Mayer, K.H. Sulfonnamides and Trimethoprim. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Philadelphia, PA, USA, 2020; ISBN 978-0-323-48255-4. [Google Scholar]
- Boxall, A.B.A.; Johnson, P.; Smith, E.J.; Sinclair, C.J.; Stutt, E.; Levy, L.S. Uptake of Veterinary Medicines from Soils into Plants. J. Agric. Food Chem. 2006, 54, 2288–2297. [Google Scholar] [CrossRef] [PubMed]
- Bhalsod, G.D.; Chuang, Y.-H.; Jeon, S.; Gui, W.; Li, H.; Ryser, E.T.; Guber, A.K.; Zhang, W. Uptake and Accumulation of Pharmaceuticals in Overhead- and Surface-Irrigated Greenhouse Lettuce. J. Agric. Food Chem. 2018, 66, 822–830. [Google Scholar] [CrossRef] [PubMed]
- ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. ISO: Geneva, Switzerland, 2017; Third Edition.
- European Commission. Commission Implementing Regulation (EU). 2021/808 of 22 March 2021 on the Performance of Analytical Methods for Residues of Pharmacologically Active Substances Used in Food-Producing Animals and on the Interpretation of Results as Well as on the Methods to Be Used for Sampling and Repealing Decisions 2002/657/EC and 98/179/EC. Off. J. Eur. Union. 2021, L 180, 84–109. [Google Scholar]
- European Medicines Agency. VICH GL2: Validation of Analytical Procedures: Methodology-Step 7. 1998 10. Available online: https://www.ema.europa.eu/en/vich-gl2-validation-analytical-procedures-methodology-scientific-guideline (accessed on 10 August 2023).
- Otzen, T.; Manterola, C. Técnicas de Muestreo Sobre Una Población a Estudio. Int. J. Morphol. 2017, 35, 227–232. [Google Scholar] [CrossRef]
- Corporación de Fomento de la Producción. CORFO. Prospección y Factibilidad Técnica, Económica y Financiera Para la Creación de un Centro de Acopio y Comercialización de Horticultura en Chile; Corporación de Fomento de la Producción: Chile, Santiago, 2017; pp. 1–75. [Google Scholar]
- World Health Organization. WHO. Critically Important Antimicrobials for Human Medicine, 6th ed.; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151552-8. [Google Scholar]
- Sallach, J.B.; Snow, D.; Hodges, L.; Li, X.; Bartelt-Hunt, S. Development and Comparison of Four Methods for the Extraction of Antibiotics from a Vegetative Matrix. Enviro. Toxic. Chem. 2016, 35, 889–897. [Google Scholar] [CrossRef] [PubMed]
- George, T.; Brady, M.F. Ethylenediaminetetraacetic Acid (EDTA). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Guzmán-Morales, A.R.; Cruz-La Paz, O.; Valdés-Carmenate, R.; Valdés-Hernández, P.A. Evaluation of Heavy Metal Contamination and Accumulation in Lettuce (Lactuca Sativa L.) Plants. Cul. Trop. 2021, 42, e03. [Google Scholar]
- Matamoros, V.; Casas, M.E.; Pastor, E.; Tadić, Đ.; Cañameras, N.; Carazo, N.; Bayona, J.M. Effects of Tetracycline, Sulfonamide, Fluoroquinolone, and Lincosamide Load in Pig Slurry on Lettuce: Agricultural and Human Health Implications. Environ. Res. 2022, 215, 114237. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, M. Effects of Combined Pollution of Tetracycline and Sulfamethazine on Tomato Growth and Antibiotic Absorption. Agronomy 2023, 13, 762. [Google Scholar] [CrossRef]
- Ćirić, S.A.; Mitić, V.D.; Nikolić, J.S.; Ilić, M.D.; Dimitrijević, M.V.; Simonović, S.R.; Stankov Jovanović, V.P. Recent Developments in Sorbent Based Water Samples Treatments Prior GC-MS Analysis of Polycyclic Aromatic Hydrocarbons. Chem. Nais. 2018, 1, 93–129. [Google Scholar] [CrossRef]
- McMillan, J. Principles of Analytical Validation. In Proteomic Profiling and Analytical Chemistry; Elsevier: Boston, MA, USA, 2016; pp. 239–251. ISBN 978-0-444-63688-1. [Google Scholar]
- Tadić, Đ.; Matamoros, V.; Bayona, J.M. Simultaneous Determination of Multiclass Antibiotics and Their Metabolites in Four Types of Field-Grown Vegetables. Anal Bioanal. Chem. 2019, 411, 5209–5222. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Wong, C.K.C.; Chu, L.M. Distribution of Antibiotics in Wastewater-Irrigated Soils and Their Accumulation in Vegetable Crops in the Pearl River Delta, Southern China. J. Agric. Food Chem. 2014, 62, 11062–11069. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Karaolia, P.; Hapeshi, E.; Michael, C.; Fatta-Kassinos, D. Long-Term Wastewater Irrigation of Vegetables in Real Agricultural Systems: Concentration of Pharmaceuticals in Soil, Uptake and Bioaccumulation in Tomato Fruits and Human Health Risk Assessment. Water Res. 2017, 109, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.; Sun, P.; Sun, Q.; Li, X.; Yang, X.; Ji, X.; Zou, H.; Ottoson, J.; Nilsson, L.E.; Berglund, B.; et al. Presence of Antibiotic Residues in Various Environmental Compartments of Shandong Province in Eastern China: Its Potential for Resistance Development and Ecological and Human Risk. Environ. Int. 2018, 114, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Tadić, Đ.; Bleda Hernandez, M.J.; Cerqueira, F.; Matamoros, V.; Piña, B.; Bayona, J.M. Occurrence and Human Health Risk Assessment of Antibiotics and Their Metabolites in Vegetables Grown in Field-Scale Agricultural Systems. J. Hazard. Mater. 2021, 401, 123424. [Google Scholar] [CrossRef]
- Martínez, J.L. Effect of Antibiotics on Bacterial Populations: A Multi-Hierachical Selection Process. F1000 Res. 2017, 6, 51. [Google Scholar] [CrossRef]
- Chow, L.K.M.; Ghaly, T.M.; Gillings, M.R. A Survey of Sub-Inhibitory Concentrations of Antibiotics in the Environment. J. Environ. Sci. 2021, 99, 21–27. [Google Scholar] [CrossRef]
- Laureti, L.; Matic, I.; Gutierrez, A. Bacterial Responses and Genome Instability Induced by Subinhibitory Concentrations of Antibiotics. Antibiotics 2013, 2, 100–114. [Google Scholar] [CrossRef]
- Muñoz, M. Boletín de Hortalizas Frescas y Procesadas. Available online: https://www.odepa.gob.cl/publicaciones/boletines/boletin-de-hortalizas-julio-2023#:~:text=Las%20importaciones%20de%20hortalizas%20en,el%20mismo%20periodo%20de%202022 (accessed on 2 October 2023).


| Time (min) | Phase A (%) | Phase B (%) |
|---|---|---|
| 0 | 85 | 15 |
| 5 | 85 | 15 |
| 5.1 | 60 | 40 |
| 10 | 60 | 40 |
| 10.1 | 10 | 90 |
| 15 | 10 | 90 |
| 16 | 85 | 15 |
| 25 | 85 | 15 |
| Analyte | Precursor Ion (Da) | Fragmented Ion (Da) | Time (ms) | DP (v) | EP (v) | CE (v) | CXP (v) |
|---|---|---|---|---|---|---|---|
| Oxytetracycline | 461.0 461.0 | 426.0 ** 381.0 *** | 100.0 100.0 | 72.0 73.0 | 10.0 10.0 | 28.0 36.0 | 25.0 22.0 |
| 4-Epi-oxytetracycline | 461.0 461.0 | 426.0 ** 444.0 *** | 100.0 100.0 | 72.0 70.0 | 10.0 10.0 | 28.0 30.0 | 25.0 15.0 |
| Enrofloxacin | 361.0 361.0 | 317.0 ** 343.0 *** | 100.0 100.0 | 50.0 50.0 | 7.00 7.00 | 28.0 23.0 | 20.0 8.0 |
| Ciprofloxacin | 332.0 332.0 | 231.0 ** 314.0 *** | 100.0 100.0 | 56.0 56.0 | 4.5 4.5 | 47.0 28.0 | 4.0 4.0 |
| Sulfachloropyridazine | 285.2 285.2 | 156.0 ** 108.0 *** | 100.0 100.0 | 61.0 61.0 | 10.0 10.0 | 21.0 31.0 | 12.0 8.0 |
| Sulfamethazine 13C6 * | 285.3 | 124.1 | 100.0 | 71.0 | 10.0 | 31.0 | 12.0 |
| Enrofloxacin-D5 * | 365.1 | 321.0 | 100.0 | 50.0 | 7.0 | 23.0 | 8.0 |
| Tetracycline-D6 * | 451.0 | 160.0 | 100.0 | 34.0 | 10.0 | 25.0 | 30.0 |
| Analyte | R2*Average | CV (%) | Slope Average | CV (%) |
|---|---|---|---|---|
| Oxytetracycline | 0.996 | 0.095 | 0.966 | 9.51 |
| 4-Epi-oxytetracycline | 0.995 | 0.166 | 1.111 | 6.794 |
| Enrofloxacin | 0.995 | 0.38 | 0.04 | 14.928 |
| Ciprofloxacin | 0.998 | 0.11 | 0.33 | 17.693 |
| Sulfachloropyridazine | 0.993 | 0.257 | 0.04 | 3.011 |
| Analyte | Working Concentration (µg·kg−1 dw) | CV (%) Repeatability | CV (%) Reproducibility | Average Recovery (%) |
|---|---|---|---|---|
| Oxytetracycline | 1 | 6.25 | 13.87 | 93.0 |
| 5 | 1.99 | 8.01 | 99.1 | |
| 15 | 0.18 | 7.05 | 97.0 | |
| 4-Epi-oxytetracycline | 1 | 7.69 | 11.64 | 99.8 |
| 5 | 3.16 | 3.25 | 100.1 | |
| 15 | 0.27 | 0.31 | 100.0 | |
| Enrofloxacin | 5 | 9.74 | 8.65 | 110.5 |
| 15 | 5.09 | 5.64 | 94.2 | |
| 30 | 1.04 | 1.05 | 101.2 | |
| Ciprofloxacin | 5 | 11.61 | 13.02 | 101.6 |
| 15 | 6.13 | 7.41 | 99.1 | |
| 30 | 1.25 | 1.47 | 100.2 | |
| Sulfachloropyridazine | 1 | 6.62 | 14.02 | 102.1 |
| 5 | 0.82 | 4.03 | 99.4 | |
| 15 | 0.09 | 0.38 | 100.1 |
| Sample ID | OTC + 4-epi-OTC (µg·kg−1 dw) | EFX + CFX (µg·kg−1 dw) | SCP (µg·kg−1 dw) | Lettuce Variety | Market |
|---|---|---|---|---|---|
| 1 | <LOD * | <LOD | <LOD | Butter 1 | Lo Valledor |
| 2 | Traces ** | <LOD | <LOD | Butter 2 | |
| 3 | <LOD | <LOD | <LOD | Butter 3 | |
| 4 | <LOD | <LOD | <LOD | Butter 4 | |
| 5 | <LOD | <LOD | <LOD | Romaine 1 | |
| 6 | Traces | <LOD | <LOD | Romaine 2 | |
| 7 | Traces | <LOD | <LOD | Romaine 3 | |
| 8 | 16.004 | <LOD | <LOD | Romaine 4 | |
| 9 | <LOD | <LOD | <LOD | Iceberg 1 | |
| 10 | <LOD | <LOD | <LOD | Iceberg 2 | |
| 11 | Traces | <LOD | <LOD | Iceberg 3 | |
| 12 | Traces | <LOD | <LOD | Iceberg 4 | |
| 13 | <LOD | Traces | <LOD | Butter 1 | Vega Central |
| 14 | <LOD | Traces | <LOD | Butter 2 | |
| 15 | 1.384 | <LOD | <LOD | Butter 3 | |
| 16 | Traces | <LOD | <LOD | Butter 4 | |
| 17 | <LOD | Traces | <LOD | Romaine 1 | |
| 18 | <LOD | <LOD | <LOD | Romaine 2 | |
| 19 | <LOD | <LOD | <LOD | Romaine 3 | |
| 20 | <LOD | <LOD | <LOD | Romaine 4 | |
| 21 | <LOD | <LOD | <LOD | Iceberg 1 | |
| 22 | <LOD | <LOD | <LOD | Iceberg 2 | |
| 23 | Traces | <LOD | <LOD | Iceberg 3 | |
| 24 | <LOD | <LOD | <LOD | Iceberg 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yévenes, K.; Ibáñez, M.J.; Pokrant, E.; Flores, A.; Maturana, M.; Maddaleno, A.; Cornejo, J. A Suitable HPLC-MS/MS Methodology for the Detection of Oxytetracycline, Enrofloxacin, and Sulfachloropyridazine Residues in Lettuce Plants. Foods 2024, 13, 153. https://doi.org/10.3390/foods13010153
Yévenes K, Ibáñez MJ, Pokrant E, Flores A, Maturana M, Maddaleno A, Cornejo J. A Suitable HPLC-MS/MS Methodology for the Detection of Oxytetracycline, Enrofloxacin, and Sulfachloropyridazine Residues in Lettuce Plants. Foods. 2024; 13(1):153. https://doi.org/10.3390/foods13010153
Chicago/Turabian StyleYévenes, Karina, María José Ibáñez, Ekaterina Pokrant, Andrés Flores, Matías Maturana, Aldo Maddaleno, and Javiera Cornejo. 2024. "A Suitable HPLC-MS/MS Methodology for the Detection of Oxytetracycline, Enrofloxacin, and Sulfachloropyridazine Residues in Lettuce Plants" Foods 13, no. 1: 153. https://doi.org/10.3390/foods13010153