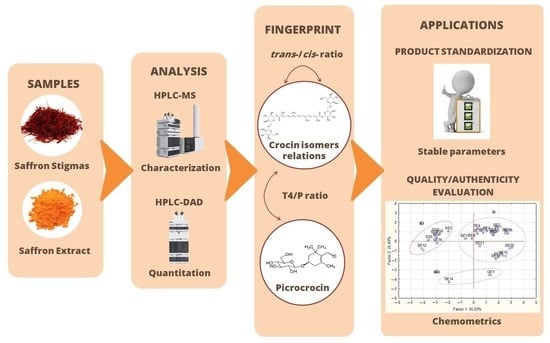
Fingerprint of Characteristic Saffron Compounds as Novel Standardization of Commercial Crocus sativus Extracts
Abstract
:1. Introduction

2. Materials and Methods
2.1. Reagents and Standards
2.2. Crocins Nomenclature
2.3. Saffron Extracts
2.4. Sample Preparation
2.5. Sample Analysis
2.5.1. Qualitative Analysis
2.5.2. Quantitative Analysis
2.5.3. Method Validation
2.6. Stability of Crocin Profile in Commercial Extracts
2.7. Statistical Analysis
3. Results and Discussion
3.1. Crocins and Picrocrocin Identification in Saffron Commercial Extract
3.2. Crocins and Picrocrocin Quantitation
3.2.1. Validation of the Method
3.2.2. Commercial Saffron Extracts
3.3. Reproducibility and Stability of Picrocrocin and Crocins Chromatographic Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moshiri, M.; Vahabzadeh, M.; Hosseinzadeh, H. Clinical Applications of Saffron (Crocus sativus) and Its Constituents: A Review. Drug Res. 2015, 65, 287–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moratalla-López, N.; Bagur, M.J.; Lorenzo, C.; Salinas, M.R.; Alonso, G.L. Bioactivity and Bioavailability of the Major Metabolites of Crocus sativus L. Flower. Molecules 2019, 24, 2827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kell, G.; Rao, A.; Beccaria, G.; Clayton, P.; Inarejos-García, A.M.; Prodanov, M. affron® a Novel Saffron Extract (Crocus sativus L.) Improves Mood in Healthy Adults over 4 Weeks in a Double-Blind, Parallel, Randomized, Placebo-Controlled Clinical Trial. Complement. Ther. Med. 2017, 33, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Drummond, P.D.; Inarejos-García, A.M.; Prodanov, M. affron®, a Standardised Extract from Saffron (Crocus sativus L.) for the Treatment of Youth Anxiety and Depressive Symptoms: A Randomised, Double-Blind, Placebo-Controlled Study. J. Affect. Disord. 2018, 232, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Husaini, A.M.; Jan, K.N.; Wani, G.A. Saffron: A Potential Drug-Supplement for Severe Acute Respiratory Syndrome Coronavirus (COVID) Management. Heliyon 2021, 7, e07068. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Katragunta, K.; Wang, Y.-H.; Upton, R.; Khan, I.A. Analysis of Crocetins and Safranal Variations in Saffron (Crocus sativus) Stigma Samples and Dietary Supplements Using HPLC/UHPLC-PDA-MS: Chemical Profiling and Chemometric Analysis Using LC-QToF. Food Anal. Methods 2022, 15, 2238–2259. [Google Scholar] [CrossRef]
- Alavizadeh, S.H.; Hosseinzadeh, H. Bioactivity Assessment and Toxicity of Crocin: A Comprehensive Review. Food Chem. Toxicol. 2014, 64, 65–80. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Kovalyov, V.; Goryacha, O.; Ivanauskas, L.; Georgiyants, V. Biologically Active Compounds and Pharmacological Activities of Species of the Genus Crocus: A Review. Phytochemistry 2019, 162, 56–89. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Fong, H.H.S.; Farnsworth, N.R. The Role of Quality Assurance and Standardization in the Safety of Botanical Dietary Supplements. Chem. Res. Toxicol. 2007, 20, 577–582. [Google Scholar] [CrossRef] [Green Version]
- Bilia, A.R.; Bergonzi, M.C. The G115 Standardized Ginseng Extract: An Example for Safety, Efficacy, and Quality of an Herbal Medicine. J. Ginseng Res. 2020, 44, 179–193. [Google Scholar] [CrossRef] [PubMed]
- AHPA (The American Herbal Products Association’s). Standardization of Botanical Products: White Paper; Botanical Extracts Committee from The American Herbal Products, Ed.; AHPA: Silver Spring, MD, USA, 2003. [Google Scholar]
- Carmona, M.; Zalacain, A.; Sánchez, A.M.; Novella, J.L.; Alonso, G.L. Crocetin Esters, Picrocrocin and Its Related Compounds Present in Crocus sativus Stigmas and Gardenia jasminoides Fruits. Tentative Identification of Seven New Compounds by LC-ESI-MS. J. Agric. Food Chem. 2006, 54, 973–979. [Google Scholar] [CrossRef] [PubMed]
- ISO. ISO. ISO 3632-2:2010 Spices-Saffron (Crocus sativus L.)—Part 2: Test Methods. In ISO International Standard; ISO: Geneva, Switzerland, 2010; pp. 1–42. [Google Scholar]
- García-Rodríguez, M.V.; López-Córcoles, H.; Alonso, G.L.; Pappas, C.S.; Polissiou, M.G.; Tarantilis, P.A. Comparative Evaluation of an ISO 3632 Method and an HPLC-DAD Method for Safranal Quantity Determination in Saffron. Food Chem. 2017, 221, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Suchareau, M.; Bordes, A.; Lemée, L. Improved Quantification Method of Crocins in Saffron Extract Using HPLC-DAD after Qualification by HPLC-DAD-MS. Food Chem. 2021, 362, 130199. [Google Scholar] [CrossRef] [PubMed]
- Moras, B.; Loffredo, L.; Rey, S. Quality Assessment of Saffron (Crocus sativus L.) Extracts via UHPLC-DAD-MS Analysis and Detection of Adulteration Using Gardenia Fruit Extract (Gardenia jasminoides Ellis). Food Chem. 2018, 257, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Girme, A.; Saste, G.; Pawar, S.; Ghule, C.; Mirgal, A.; Patel, S.; Tiwari, A.; Ghoshal, S.; Bharate, S.B.; Bharate, S.S.; et al. Quantitative Determination and Characterization of a Kashmir Saffron (Crocus sativus L.)-Based Botanical Supplement Using Single-Laboratory Validation Study by HPLC-PDA with LC-MS/MS and HPTLC Investigations. ACS Omega 2021, 6, 23460–23474. [Google Scholar] [CrossRef] [PubMed]
- García-Blázquez, A.; Moratalla-López, N.; Alonso, G.L.; Lorenzo, C.; Salinas, M.R. Effect of Crocus sativus L. Stigmas Microwave Dehydration on Picrocrocin, Safranal and Crocetin Esters. Foods 2021, 10, 404. [Google Scholar] [CrossRef]
- José Bagur, M.; Alonso Salinas, G.L.; Jiménez-Monreal, A.M.; Chaouqi, S.; Llorens, S.; Martínez-Tomé, M.; Alonso, G.L. Saffron: An Old Medicinal Plant and a Potential Novel Functional Food. Molecules 2017, 23, 30. [Google Scholar] [CrossRef] [Green Version]
- Rocchi, R.; Mascini, M.; Sergi, M.; Compagnone, D.; Mastrocola, D.; Pittia, P. Crocins Pattern in Saffron Detected by UHPLC-MS/MS as Marker of Quality, Process and Traceability. Food Chem. 2018, 264, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Guijarro-Díez, M.; Castro-Puyana, M.; Crego, A.L.; Marina, M.L. Detection of Saffron Adulteration with Gardenia Extracts through the Determination of Geniposide by Liquid Chromatography–Mass Spectrometry. J. Food Compos. Anal. 2017, 55, 30–37. [Google Scholar] [CrossRef]
- Cid-Pérez, T.S.; Nevárez-Moorillón, G.V.; Ochoa-Velasco, C.E.; Navarro-Cruz, A.R.; Hernández-Carranza, P.; Avila-Sosa, R. The Relation between Drying Conditions and the Development of Volatile Compounds in Saffron (Crocus sativus). Molecules 2021, 26, 6954. [Google Scholar] [CrossRef]
- Caballero-Ortega, H.; Pereda-Miranda, R.; Abdullaev, F.I. HPLC Quantification of Major Active Components from 11 Different Saffron (Crocus sativus L.) Sources. Food Chem. 2007, 100, 1126–1131. [Google Scholar] [CrossRef]
- Almodóvar, P.; Briskey, D.; Rao, A.; Prodanov, M.; Inarejos-García, A.M. Bioaccessibility and Pharmacokinetics of a Commercial Saffron (Crocus sativus L.) Extract. Evid. Based Complement. Altern. Med. 2020, 2020, 575730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ICH. Validation of Analytical Procedures: Text and Methodology Q2 (R1); ICH: Geneva, Switzerland, 2005. [Google Scholar]
- García-Rodríguez, M.V.; Serrano-Díaz, J.; Tarantilis, P.A.; López-Córcoles, H.; Carmona, M.; Alonso, G.L. Determination of Saffron Quality by High-Performance Liquid Chromatography. J. Agric. Food Chem. 2014, 62, 8068–8074. [Google Scholar] [CrossRef]
- Bharate, S.S.; Kumar, V.; Singh, G.; Singh, A.; Gupta, M.; Singh, D.; Kumar, A.; Vishwakarma, R.A.; Bharate, S.B. Preclinical Development of Crocus sativus-Based Botanical Lead IIIM-141 for Alzheimer’s Disease: Chemical Standardization, Efficacy, Formulation Development, Pharmacokinetics, and Safety Pharmacology. ACS Omega 2018, 3, 9572–9585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Archivio, A.; Di Donato, F.; Foschi, M.; Maggi, M.; Ruggieri, F. UHPLC Analysis of Saffron (Crocus sativus L.): Optimization of Separation Using Chemometrics and Detection of Minor Crocetin Esters. Molecules 2018, 23, 1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Chen, N.; Yang, J.; Yang, B.; Ouyang, Z.; Wu, C.; Yuan, Y.; Wang, W.; Chen, M. An Integrated Approach Combining HPLC, GC/MS, NIRS, and Chemometrics for the Geographical Discrimination and Commercial Categorization of Saffron. Food Chem. 2018, 253, 284–292. [Google Scholar] [CrossRef]
- Tabtabaei, S.; D’Archivio, A.A.; Maggi, M.A.; Brutus, M.; Bajracharya, D.H.; Konakbayeva, D.; Soleimani, A.; Brim, H.; Ashktorab, H. Geographical Classification of Iranian and Italian Saffron Sources Based on HPLC Analysis and UV–Vis Spectra of Aqueous Extracts. Eur. Food Res. Technol. 2019, 245, 2435–2446. [Google Scholar] [CrossRef]
- Razavizadeh, B.M.; Arabshahi Delooei, N. Quantification of Crocin, Picrocrocin and Safranal in Saffron Stigmas Obtained from Sounded Corms with Acoustic Waves. Phytochem. Anal. 2021, 32, 1059–1066. [Google Scholar] [CrossRef]
- Lech, K.; Witowska-Jarosz, J.; Jarosz, M. Saffron Yellow: Characterization of Carotenoids by High Performance Liquid Chromatography with Electrospray Mass Spectrometric Detection. J. Mass Spectrom. 2009, 44, 1661–1667. [Google Scholar] [CrossRef]
- Verma, R.S.; Middha, D. Analysis of Saffron (Crocus sativus L. Stigma) Components by LC–MS–MS. Chromatographia 2010, 71, 117–123. [Google Scholar] [CrossRef]
- Pittenauer, E.; Koulakiotis, N.S.; Tsarbopoulos, A.; Allmaier, G. In-Chain Neutral Hydrocarbon Loss from Crocin Apocarotenoid Ester Glycosides and the Crocetin Aglycon (Crocus sativus L.) by ESI-MSn (n = 2, 3). J. Mass Spectrom. 2013, 48, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Pittenauer, E.; Rados, E.; Tsarbopoulos, A.; Allmaier, G. In-Depth Analysis of Crocetin Ester Glycosides from Dried/Processed Stigmas of Crocus sativus L. by HPLC-ESI-MSn(n = 2, 3). Phytochem. Anal. 2019, 30, 346–356. [Google Scholar] [CrossRef]
- Hegazi, N.M.; Khattab, A.R.; Frolov, A.; Wessjohann, L.A.; Farag, M.A. Authentication of Saffron Spice Accessions from Its Common Substitutes via a Multiplex Approach of UV/VIS Fingerprints and UPLC/MS Using Molecular Networking and Chemometrics. Food Chem. 2022, 367, 130739. [Google Scholar] [CrossRef]
- Cossignani, L.; Urbani, E.; Simonetti, M.S.; Maurizi, A.; Chiesi, C.; Blasi, F. Characterisation of Secondary Metabolites in Saffron from Central Italy (Cascia, Umbria). Food Chem. 2014, 143, 446–451. [Google Scholar] [CrossRef]
- Karkoula, E.; Angelis, A.; Koulakiotis, N.S.; Gikas, E.; Halabalaki, M.; Tsarbopoulos, A.; Skaltsounis, A.L. Rapid Isolation and Characterization of Crocins, Picrocrocin, and Crocetin from Saffron Using Centrifugal Partition Chromatography and LC–MS. J. Sep. Sci. 2018, 41, 4105–4114. [Google Scholar] [CrossRef]
- Speranza, G.; Dadà, G.; Manitto, P.; Monti, D.; Gramatica, P. 13-Cis Crocin: A New Crocinoid of Saffron. Gazz. Chim. Ital. 1984, 114, 189–192. [Google Scholar]
- Carmona, M.; Zalacain, A.; Pardo, J.E.; López, E.; Alvarruiz, A.; Alonso, G.L. Influence of Different Drying and Aging Conditions on Saffron Constituents. J. Agric. Food Chem. 2005, 53, 3974–3979. [Google Scholar] [CrossRef] [PubMed]
- Alonso, G.L.; Salinas, M.R.; Garijo, J.; Sánchez-Fernández, M.A. Composition of Crocins and Picrocrocin from Spanish Saffron (Crocus sativus L.). J. Food Qual. 2001, 24, 219–233. [Google Scholar] [CrossRef]
- Sereshti, H.; Ataolahi, S.; Aliakbarzadeh, G.; Zarre, S.; Poursorkh, Z. Evaluation of Storage Time Effect on Saffron Chemical Profile Using Gas Chromatography and Spectrophotometry Techniques Coupled with Chemometrics. J. Food Sci. Technol. 2018, 55, 1350–1359. [Google Scholar] [CrossRef] [PubMed]





| Nº | Compound | Molecular Formula | Mw | Detected m/z Ions | Fragmentation (m/z) |
|---|---|---|---|---|---|
| 1 | trans-5-tG | C50H74O29 | 1138.4316 | 1156.4654 [M+NH4]+ | 832.3602 [M+NH4-G]+; 653.2805 [M+H-t]+; 329.1749 [M+H-t-G]+ |
| 2 | trans-5-nG | C50H74O29 | 1138.4316 | 1156.4654 [M+NH4]+ | 832.3602 [M+NH4-G]+; 653.2805 [M+H-n]+; 329.1749 [M+H-n-G]+ |
| 3 | trans-4-GG | C44H64O24 | 976.3788 | 994.4212 [M+NH4]+ | 653.2758 [M+H-G]+; 635.2687 [M+H-G-H2O]+; 329.1776 [M+H-2G]+; 311.16729 [M+H-2G-H2O]+; 293.1538 [M+H-2G-2H2O]+ |
| 4 | trans-4-tg | C44H64O24 | 976.3788 | 994.4212 [M+NH4]+ | 508.1736 [M+NH4-t]+; 329.1749 [M+H-t-g]+ |
| 5 | trans-4-ng | C44H64O24 | 976.3788 | 994.4212 [M+NH4]+ | 508.1736 [M+NH4-n]+; 491.1191 [M+H-n]+; 329.1749 [M+H-n-g]+ |
| 6 | trans-3-Gg | C38H54O19 | 814.3259 | 832.3598 [M+NH4]+ | 670.3051 [M+NH4-g]+; 329.1753 [M+H-g-G]+; 311.1649 [M+H-g-G-H2O]+; 293.1538 [M+H-g-G-2H2O]+ |
| 7 | trans-2-gg | C32H44O14 | 652.2731 | 670.3069 [M+NH4]+ | 329.1753 [M+H-gg]+; 311.1649 [M+H-gg-H2O]+; 293.1538 [M+H-gg-2H2O]+ |
| 8 | cis-4-GG | C44H64O24 | 976.3788 | 994.4212 [M+NH4]+ | 670.3052 [M+NH4-G]+; 635.2687 [M+H-G-H2O]+; 329.1776 [M+H-2G]+; 311.16729 [M+H-2G-H2O]+; 293.1538 [M+H-2G-2H2O]+ |
| 9 | cis-4-ng | C44H64O24 | 976.3788 | 994.4212 [M+NH4]+ | 653.2758 [M+H-G]+; 635.2687 [M+H-G-H2O]+; 508.1736 [M+NH4-n]+; 329.1776 [M+H-2G]+; 311.16729 [M+H-2G-H2O]+; 293.1538 [M+H-2G-2H2O]+ |
| 10 | trans-2-G | C32H44O14 | 652.2731 | 670.3069 [M+NH4]+ | 329.1753 [M+H-G]+; 311.1649 [M+H-G-H2O]+; 293.1538 [M+H-G-2H2O]+ |
| 11 | cis-3-Gg | C38H54O19 | 814.3259 | 832.3598 [M+NH4]+ | 670.3051 [M+NH4-g]+; 329.1753 [M+H-g-G]+; 311.1649 [M+H-g-G-H2O]+; 293.1538 [M+H-g-G-2H2O]+ |
| 12 | cis-2-gg | C32H44O14 | 652.2731 | 670.3069 [M+NH4]+ | 329.1753 [M+H-gg]+; 311.1649 [M+H-gg-H2O]+; 293.1538 [M+H-gg-2H2O]+ |
| 13 | trans-1-g | C26H34O9 | 490.2203 | 513.2112 [M+Na]+ | 329.1753 [M+H-g]+; 311.1649 [M+H-g-H2O]+; 293.1538 [M+H-g-2H2O]+ |
| 14 | cis-2-G | C32H44O14 | 652.2731 | 670.3069 [M+NH4]+ | 329.1753 [M+H-G]+; 311.1649 [M+H-G-H2O]+; 293.1538 [M+H-G-2H2O]+ |
| 15 | cis-1-g | C26H34O9 | 490.2203 | 513.2112 [M+Na]+ | 329.1753 [M+H-g]+; 311.1649 [M+H-g-H2O]+; 293.1538 [M+H-g-2H2O]+ |
| 16 | trans-crocetin | C20H24O4 | 328.1675 | 329.1741 [M+H]+ | 311.16729 [M+H-H2O]+; 293.1538 [M+H-2H2O]+ |
| 17 | cis-crocetin | C20H24O4 | 328.1675 | 329.1741 [M+H]+ | 311.16729 [M+H-H2O]+; 293.1538 [M+H-2H2O]+ |
| Crocins | Picrocrocin | |
|---|---|---|
| Calibration curve | y = 157.9x − 305.9 (R2 = 0.9991) | y = 79.44x − 59.91 (R2 = 0.9998) |
| Linear range (µg mL−1) | 0.93–232.43 | 18.24–243.26 |
| Repeatability (RSD %) | 2.03 a/2.14 b | 5.54 a/1.77 b |
| Intermediate precision (RSD %) | 3.04 | 2.55 |
| LOD (µg mL−1) | 0.13 | 3.48 |
| LOQ (µg mL−1) | 0.40 | 10.54 |
| Recovery (%) | 95 ± 2 | 99 ± 2 |
| ID | % | trans- 5-tG | trans- 4-GG | trans- 4-tg | trans- 3-Gg | cis- 4-GG | cis- 3-Gg | trans- 2-G | Total trans- | Total cis- | trans/cis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SE1 | Mean | 0.8 | 45.17 | 3.1 | 17.1 | 8.0 | 4.2 | 8.5 | 83.44 | 16.6 | 5.0 |
| RSD | 0.3 | 0.04 | 0.7 | 0.5 | 0.4 | 2.0 | 0.9 | 0.02 | 0.1 | 0.1 | |
| SE2 | Mean | 0.9 | 46.0 | 2.4 | 17.6 | 7.8 | 2.9 | 8.8 | 83.3 | 16.6 | 5.0 |
| RSD | 2.5 | 0.4 | 1.6 | 0.4 | 0.5 | 2.8 | 2.4 | 0.2 | 0.6 | 0.5 | |
| SE3 | Mean | 0.9 | 47.3 | 2.2 | 18.1 | 7.7 | 3.6 | 8.9 | 85.3 | 14.7 | 5.8 |
| RSD | 0.5 | 1.0 | 2.6 | 0.7 | 1.7 | 3.8 | 2.4 | 0.5 | 2.8 | 3.3 | |
| SE4 | Mean | 1.0 | 48.0 | 1.6 | 18.5 | 7.8 | 3.3 | 8.0 | 85.6 | 14.4 | 5.9 |
| RSD | 2.2 | 0.5 | 0.8 | 0.2 | 1.0 | 5.8 | 2.9 | 0.1 | 0.4 | 0.4 | |
| SE5 | Mean | 0.9 | 47.2 | 1.3 | 18.4 | 8.1 | 3.0 | 9.3 | 84.7 | 15.3 | 5.5 |
| RSD | 2.1 | 0.2 | 0.6 | 0.3 | 0.2 | 1.4 | 3.7 | 1.0 | 5.5 | 6.5 | |
| SE6 | Mean | 0.9 | 49.1 | 2.0 | 19.5 | 6.8 | 2.7 | 7.8 | 86.5 | 13.5 | 6.4 |
| RSD | 1.1 | 0.3 | 1.7 | 0.8 | 0.1 | 2.8 | 0.5 | 0.2 | 1.2 | 1.4 | |
| SE7 | Mean | 0.8 | 44.6 | 2.2 | 16.9 | 7.9 | 4.6 | 10.1 | 84.00 | 16.0 | 5.3 |
| RSD | 0.5 | 0.3 | 0.2 | 0.8 | 0.3 | 5.2 | 3.9 | 0.04 | 0.2 | 0.2 | |
| SE8 | Mean | 0.87 | 45.8 | 2.5 | 17.4 | 7.9 | 2.97 | 8.1 | 83.1 | 16.9 | 4.9 |
| RSD | 0.03 | 0.2 | 0.9 | 0.5 | 0.1 | 0.02 | 0.4 | 0.1 | 0.7 | 0.8 | |
| SE9 | Mean | 0.9 | 46.0 | 1.7 | 17.6 | 8.1 | 3.0 | 9.0 | 83.3 | 16.7 | 5.0 |
| RSD | 0.2 | 0.2 | 1.1 | 0.3 | 0.1 | 0.9 | 0.6 | 0.1 | 0.6 | 0.7 | |
| SE10 | Mean | 0.9 | 48.1 | 1.6 | 18.8 | 7.5 | 2.8 | 7.5 | 85.2 | 14.8 | 5.8 |
| RSD | 0.4 | 0.3 | 0.5 | 0.8 | 1.2 | 0.5 | 0.5 | 0.1 | 0.4 | 0.4 | |
| SE11 | Mean | 0.8 | 43.9 | 2.4 | 16.9 | 8.8 | 3.1 | 10.1 | 82.0 | 18.0 | 4.5 |
| RSD | 0.6 | 1.3 | 2.4 | 1.3 | 3.4 | 0.3 | 0.3 | 0.9 | 4.3 | 5.2 | |
| SE12 | Mean | 1.1 | 55.8 | 0.2 | 22.94 | 3.4 | 1.4 | 6.1 | 92.53 | 7.5 | 12.4 |
| RSD | 5.0 | 0.2 | 2.3 | 0.04 | 2.2 | 0.6 | 0.8 | 0.04 | 0.5 | 0.6 | |
| SE13 | Mean | 0.9 | 42.4 | 2.8 | 16.14 | 8.0 | 3.1 | 9.7 | 81.1 | 18.9 | 4.3 |
| RSD | 0.1 | 0.2 | 0.2 | 0.04 | 0.4 | 2.2 | 1.4 | 0.2 | 0.8 | 1.0 | |
| SE14 | Mean | 0.4 | 64.7 | 1.6 | 8.6 | 4.6 | 0.8 | 5.5 | 91.45 | 8.5 | 10.7 |
| RSD | 1.9 | 0.2 | 2.3 | 0.1 | 0.1 | 0.8 | 1.1 | 0.02 | 0.2 | 0.2 | |
| SE15 | Mean | 0.8 | 41.98 | 5.7 | 14.9 | 6.1 | 2.2 | 10.6 | 84.4 | 15.6 | 5.4 |
| RSD | 0.6 | 0.01 | 0.1 | 0.5 | 2.8 | 1.0 | 7.7 | 0.2 | 0.9 | 1.1 | |
| SE16 | Mean | 1.0 | 55.1 | 0.2 | 21.6 | 5.0 | 2.0 | 6.5 | 90.7 | 9.3 | 9.8 |
| RSD | 0.6 | 0.1 | 14.6 | 0.1 | 1.1 | 0.5 | 4.2 | 0.1 | 1.4 | 1.6 | |
| SE17 | Mean | 1.0 | 49.5 | 1.0 | 19.5 | 6.2 | 2.4 | 7.9 | 86.6 | 13.4 | 6.5 |
| RSD | 0.6 | 0.5 | 2.7 | 0.1 | 0.3 | 1.4 | 2.2 | 0.2 | 1.2 | 1.4 | |
| SE18 | Mean | 0.8 | 36.5 | 3.4 | 22.0 | 5.5 | 0.9 | 12.6 | 84.17 | 15.8 | 5.3 |
| RSD | 0.0 | 0.1 | 0.2 | 0.6 | 0.6 | 0.3 | 1.1 | 0.02 | 0.1 | 0.1 | |
| SE19 | Mean | 0.7 | 36.1 | 3.4 | 23.8 | 5.8 | 0.8 | 12.9 | 85.5 | 14.5 | 5.9 |
| RSD | 0.5 | 0.8 | 1.1 | 0.6 | 0.5 | 0.6 | 0.1 | 0.2 | 1.2 | 1.4 | |
| SE20 | Mean | 0.7 | 39.2 | 3.2 | 19.1 | 7.3 | 1.4 | 11.4 | 81.1 | 18.9 | 4.3 |
| RSD | 0.6 | 1.2 | 6.3 | 1.1 | 0.2 | 1.6 | 0.1 | 0.7 | 3.2 | 3.9 | |
| SE21 | Mean | 1.0 | 42.75 | 2.5 | 21.7 | 5.8 | 1.2 | 9.4 | 84.9 | 15.1 | 5.6 |
| RSD | 0.3 | 0.01 | 1.3 | 0.7 | 0.8 | 1.1 | 0.9 | 0.1 | 0.5 | 0.5 | |
| SS1 | Mean | 1.1 | 57.1 | 0.6 | 23.2 | 6.0 | 2.4 | 5.7 | 91.05 | 9.0 | 10.2 |
| RSD | 0.9 | 0.2 | 1.4 | 0.4 | 0.5 | 0.2 | 1.2 | 0.03 | 0.3 | 0.3 | |
| SS2 | Mean | 1.2 | 57.2 | 0.5 | 23.1 | 6.2 | 2.4 | 5.7 | 90.77 | 9.2 | 9.8 |
| RSD | 0.8 | 0.0 | 1.1 | 0.1 | 0.1 | 0.1 | 0.5 | 0.01 | 0.1 | 0.1 | |
| SS3 | Mean | 1.0 | 55.9 | 0.5 | 23.0 | 7.2 | 2.9 | 5.8 | 89.17 | 10.8 | 8.2 |
| RSD | 0.2 | 0.1 | 0.1 | 0.2 | 0.3 | 0.2 | 0.5 | 0.02 | 0.2 | 0.2 | |
| SS4 | Mean | 1.0 | 57.5 | 0.6 | 23.4 | 6.1 | 2.4 | 5.5 | 90.9 | 9.1 | 10.0 |
| RSD | 0.8 | 0.3 | 2.5 | 0.3 | 0.2 | 0.0 | 0.3 | 0.0 | 0.3 | 0.3 | |
| SS5 | Mean | 1.0 | 57.9 | 0.6 | 24.7 | 5.4 | 2.2 | 4.8 | 91.9 | 8.1 | 11.4 |
| RSD | 0.2 | 0.3 | 1.5 | 0.2 | 1.2 | 1.6 | 0.3 | 0.1 | 1.4 | 1.5 | |
| SS6 | Mean | 1.0 | 56.67 | 0.6 | 24.1 | 6.5 | 2.8 | 5.2 | 90.2 | 9.8 | 9.2 |
| RSD | 0.4 | 0.04 | 1.5 | 0.1 | 1.5 | 0.9 | 2.7 | 0.1 | 1.1 | 1.2 | |
| GE1 | Mean | 0.4 | 57.0 | 4.5 | 10.9 | 7.4 | 0.9 | 9.8 | 87.9 | 12.1 | 7.3 |
| RSD | 0.8 | 0.3 | 0.5 | 1.0 | 0.9 | 1.7 | 0.6 | 0.5 | 3.8 | 4.3 |
| ID | Picrocrocin | T4/P | |
|---|---|---|---|
| SE1 | Mean | 35.3 | 0.57 |
| RSD | 1.05 | 1.24 | |
| SE2 | Mean | 32.3 | 0.62 |
| RSD | 0.79 | 0.6 | |
| SE3 | Mean | 32.1 | 0.61 |
| RSD | 0.18 | 1.77 | |
| SE4 | Mean | 33.2 | 0.67 |
| RSD | 0.34 | 0.96 | |
| SE5 | Mean | 35.0 | 0.62 |
| RSD | 0.47 | 0.74 | |
| SE6 | Mean | 30.7 | 0.70 |
| RSD | 0.66 | 0.72 | |
| SE7 | Mean | 35.2 | 0.57 |
| RSD | 0.22 | 0.19 | |
| SE8 | Mean | 33.2 | 0.61 |
| RSD | 0.36 | 1.03 | |
| SE9 | Mean | 33.8 | 0.61 |
| RSD | 1.29 | 1.03 | |
| SE10 | Mean | 33.2 | 0.65 |
| RSD | 0.05 | 0.28 | |
| SE11 | Mean | 29.6 | 0.53 |
| RSD | 1.92 | 4.11 | |
| SE12 | Mean | 140.4 | 0.71 |
| RSD | 1.02 | 2.1 | |
| SE13 | Mean | 25.8 | 0.39 |
| RSD | 1.39 | 0.41 | |
| SE14 | Mean | ND | ND |
| RSD | ND | ND | |
| SE15 | Mean | 26.4 | 0.54 |
| RSD | 17.01 | 0.69 | |
| SE16 | Mean | 5.1 | 0.86 |
| RSD | 1.74 | 1.76 | |
| SE17 | Mean | 39.7 | 0.80 |
| RSD | 0.01 | 0.94 | |
| SE18 | Mean | 20.5 | 0.67 |
| RSD | 0.77 | 0.12 | |
| SE19 | Mean | 15.4 | 0.81 |
| RSD | 2.25 | 0.64 | |
| SE20 | Mean | 25.8 | 0.79 |
| RSD | 0.03 | 0.004 | |
| SE21 | Mean | 23.7 | 0.96 |
| RSD | 0.16 | 0.45 | |
| SS1 | Mean | 102.0 | 0.81 |
| RSD | 0.09 | 0.56 | |
| SS2 | Mean | 91.6 | 0.97 |
| RSD | 0.09 | 0.25 | |
| SS3 | Mean | 89.0 | 0.97 |
| RSD | 3.23 | 0.1 | |
| SS4 | Mean | 105.4 | 0.91 |
| RSD | 2.77 | 0.86 | |
| SS5 | Mean | 112.5 | 0.84 |
| RSD | 4.02 | 1.66 | |
| SS6 | Mean | 119.9 | 0.73 |
| RSD | 0.09 | 1.82 | |
| GE1 | Mean | ND | ND |
| RSD | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mena-García, A.; Herrero-Gutiérrez, D.; Sanz, M.L.; Díez-Municio, M.; Ruiz-Matute, A.I. Fingerprint of Characteristic Saffron Compounds as Novel Standardization of Commercial Crocus sativus Extracts. Foods 2023, 12, 1634. https://doi.org/10.3390/foods12081634
Mena-García A, Herrero-Gutiérrez D, Sanz ML, Díez-Municio M, Ruiz-Matute AI. Fingerprint of Characteristic Saffron Compounds as Novel Standardization of Commercial Crocus sativus Extracts. Foods. 2023; 12(8):1634. https://doi.org/10.3390/foods12081634
Chicago/Turabian StyleMena-García, Adal, Diego Herrero-Gutiérrez, María L. Sanz, Marina Díez-Municio, and Ana I. Ruiz-Matute. 2023. "Fingerprint of Characteristic Saffron Compounds as Novel Standardization of Commercial Crocus sativus Extracts" Foods 12, no. 8: 1634. https://doi.org/10.3390/foods12081634