Chitosan Coating Functionalized with Flaxseed Oil and Green Tea Extract as a Bio-Based Solution for Beef Preservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Beef Samples
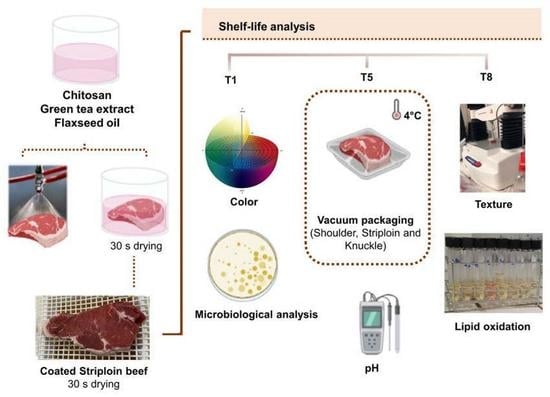
2.3. Experimental Design and Coating Preparation
2.4. Characterization of Coating Formulations
2.4.1. pH and Contact Angle Measurements
2.4.2. Rheological Parameters
Flow Curves
Oscillatory Measurements
2.5. Application of Optimized Coating Formulation on Beef Cut Samples
2.6. Shelf-Life Analysis
2.6.1. Microbiological Analysis
2.6.2. Mechanical Properties
2.6.3. Colorimetric Parameters
2.6.4. pH Value
2.6.5. Lipid Oxidation (TBARS)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Selection of Coating Formulations for Beef Application
3.1.1. pH and Contact Angle
3.1.2. Rheological Parameters
3.2. Shelf-Life Evaluation
3.2.1. Microbiological Analysis
3.2.2. Textural Properties
3.2.3. Colorimetric Parameters
3.2.4. pH Value
3.2.5. Lipid Oxidation (TBARS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schumann, B.; Schmid, M. Packaging Concepts for Fresh and Processed Meat—Recent Progresses. Innov. Food Sci. Emerg. Technol. 2018, 47, 88–100. [Google Scholar] [CrossRef]
- Soro, A.B.; Noore, S.; Hannon, S.; Whyte, P.; Bolton, D.J.; O’Donnell, C.; Tiwari, B.K. Current Sustainable Solutions for Extending the Shelf Life of Meat and Marine Products in the Packaging Process. Food Packag. Shelf Life 2021, 29, 100722. [Google Scholar] [CrossRef]
- Sánchez-Ortega, I.; García-Almendárez, B.E.; Santos-López, E.M.; Amaro-Reyes, A.; Barboza-Corona, J.E.; Regalado, C. Antimicrobial Edible Films and Coatings for Meat and Meat Products Preservation. Sci. World J. 2014, 2014, 248935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible Films/Coating with Tailored Properties for Active Packaging of Meat, Fish and Derived Products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Aminzare, M.; Hashemi, M.; Ansarian, E.; Bimkar, M.; Azar, H.H.; Mehrasbi, M.R.; Daneshamooz, S.; Raeisi, M.; Jannat, B.; Afshari, A. Using Natural Antioxidants in Meat and Meat Products as Preservatives: A Review. Adv. Anim. Vet. Sci. 2019, 7, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Gil, M.; Rudy, M. Innovations in the Packaging of Meat and Meat Products—A Review. Coatings 2023, 13, 333. [Google Scholar] [CrossRef]
- McMillin, K.W. Advancements in Meat Packaging. Meat Sci. 2017, 132, 153–162. [Google Scholar] [CrossRef]
- Vital, A.C.P.; Guerrero, A.; Monteschio, J.D.O.; Valero, M.V.; Carvalho, C.B.; de Abreu Filho, B.A.; Madrona, G.S.; do Prado, I.N. Effect of Edible and Active Coating (with Rosemary and Oregano Essential Oils) on Beef Characteristics and Consumer Acceptability. PLoS ONE 2016, 11, e0160535. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, S.; Vital, A.C.P.; Mottin, C.; do Prado, R.M.; Ornaghi, M.G.; Ramos, T.R.; Guerrero, A.; Pilau, E.J.; do Prado, I.N. Use of Alginate Edible Coating and Basil (Ocimum Spp.) Extracts on Beef Characteristics during Storage. J. Food Sci. Technol. 2021, 58, 3835–3843. [Google Scholar] [CrossRef]
- Rahman, S.M.E.; Islam, S.M.A.; Xi, Q.; Han, R.; Oh, D.H.; Wang, J. Control of Bacterial Biofilms in Red Meat—A Systematic Review. Meat Sci. 2022, 192, 108870. [Google Scholar] [CrossRef]
- Aminzare, M.; Hashemi, M.; Hassanzad Azar, H.; Hejazi, J. The Use of Herbal Extracts and Essential Oils as a Potential Antimicrobial in Meat and Meat Products; A Review. J. Hum. Environ. Health Promot. 2016, 1, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Smaoui, S.; Ben Hlima, H.; Tavares, L.; Ben Braïek, O.; Ennouri, K.; Abdelkafi, S.; Mellouli, L.; Mousavi Khaneghah, A. Application of Eco-Friendly Active Films and Coatings Based on Natural Antioxidant in Meat Products: A Review. Prog. Org. Coat. 2022, 166, 106780. [Google Scholar] [CrossRef]
- Song, D.H.; Hoa, V.B.; Kim, H.W.; Khang, S.M.; Cho, S.H.; Ham, J.S.; Seol, K.H. Edible Films on Meat and Meat Products. Coatings 2021, 11, 1344. [Google Scholar] [CrossRef]
- Bhagath, Y.B.; Manjula, K. Influence of Composite Edible Coating Systems on Preservation of Fresh Meat Cuts and Products: A Brief Review on Their Trends and Applications. Artic. Int. Food Res. J. 2019, 26, 377–392. [Google Scholar]
- Zhang, M.; Biesold, G.M.; Choi, W.; Yu, J.; Deng, Y.; Silvestre, C.; Lin, Z. Recent Advances in Polymers and Polymer Composites for Food Packaging. Mater. Today 2022, 53, 134–161. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Noipha, S. Active Film from Chitosan Incorporating Green Tea Extract for Shelf Life Extension of Pork Sausages. Food Hydrocoll. 2012, 27, 102–108. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of Plant Extracts to Meat and Meat Products to Extend Shelf-Life and Health-Promoting Attributes: An Overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Amadio, C.; Farrando, S.; Zimmermann, M.; Amadio, C.; Farrando, S.; Zimmermann, M. Effect of Chitosan Coating Enriched with Oregano Essential Oil on the Quality of Refrigerated Meat Hamburgers. Rev. FCA UNCuyo 2019, 51, 173–189. [Google Scholar]
- Mirsharifi, S.M.; Sami, M.; Jazaeri, M.; Rezaei, A. Production, Characterization, and Antimicrobial Activity of Almond Gum/Polyvinyl Alcohol/Chitosan Composite Films Containing Thyme Essential Oil Nanoemulsion for Extending the Shelf-Life of Chicken Breast Fillets. Int. J. Biol. Macromol. 2023, 227, 405–415. [Google Scholar] [CrossRef]
- Rizvi, Q.-H.; Shams, R.; Pandey, V.K.; Dar, A.H.; Tripathi, A. A Descriptive Review on Nutraceutical Constituents, Detoxification Methods and Potential Health Benefits of Flaxseed. Appl. Food Res. 2022, 2, 100239. [Google Scholar] [CrossRef]
- Higueras, L.; López-Carballo, G.; Hernández-Muñoz, P.; Gavara, R.; Rollini, M. Development of a Novel Antimicrobial Film Based on Chitosan with LAE (Ethyl-Nα-Dodecanoyl-l-Arginate) and Its Application to Fresh Chicken. Int. J. Food Microbiol. 2013, 165, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Li, B.; Liu, Y.; Wu, D.; Bai, Y.; Xiang, Q. Effect of Chitosan Coating Incorporated with Oregano Essential Oil on Microbial Inactivation and Quality Properties of Refrigerated Chicken Breasts. LWT 2023, 176, 114547. [Google Scholar] [CrossRef]
- Petrou, S.; Tsiraki, M.; Giatrakou, V.; Savvaidis, I.N. Chitosan Dipping or Oregano Oil Treatments, Singly or Combined on Modified Atmosphere Packaged Chicken Breast Meat. Int. J. Food Microbiol. 2012, 156, 264–271. [Google Scholar] [CrossRef]
- Shan, P.; Wang, K.; Yu, F.; Yi, L.; Sun, L.; Li, H. Gelatin/Sodium Alginate Multilayer Composite Film Crosslinked with Green Tea Extract for Active Food Packaging Application. Colloids Surf. A Phys. Eng. Asp. 2023, 662, 131013. [Google Scholar] [CrossRef]
- Andrade, M.A.; Barbosa, C.H.; Cerqueira, M.A.; Azevedo, A.G.; Barros, C.; Machado, A.V.; Coelho, A.; Furtado, R.; Correia, C.B.; Saraiva, M.; et al. PLA Films Loaded with Green Tea and Rosemary Polyphenolic Extracts as an Active Packaging for Almond and Beef. Food Packag. Shelf Life 2023, 36, 101041. [Google Scholar] [CrossRef]
- Vieira, J.M.; Flores-López, M.L.; de Rodríguez, D.J.; Sousa, M.C.; Vicente, A.A.; Martins, J.T. Effect of Chitosan-Aloe Vera Coating on Postharvest Quality of Blueberry (Vaccinium corymbosum) Fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Ruiz De Huidobro, F.; Miguel, E.; Blázquez, B.; Onega, E. A Comparison between Two Methods (Warner-Bratzler and Texture Profile Analysis) for Testing Either Raw Meat or Cooked Meat. Meat Sci. 2005, 69, 527–536. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Hassanein, W.S.; Alkabaa, A.S.; Ceylan, Z. Electrospun Eugenol-Loaded Gelatin Nanofibers as Bioactive Packaging Materials to Preserve Quality Characteristics of Beef. Food Packag. Shelf Life 2022, 34, 100968. [Google Scholar] [CrossRef]
- Badar, I.H.; Li, Y.; Liu, H.; Chen, Q.; Liu, Q.; Kong, B. Effect of Vegetable Oil Hydrogel Emulsion as a Fat Substitute on the Physicochemical Properties, Fatty Acid Profile, and Color Stability of Modified Atmospheric Packaged Buffalo Burgers. Meat Sci. 2023, 199, 109143. [Google Scholar] [CrossRef]
- Knecht, D.; Duziński, K.; Jankowska-Makosa, A. Bloom Time Effect Depends on Muscle Type and May Determine the Results of Ph and Color Instrumental Evaluation. Animals 2021, 11, 1282. [Google Scholar] [CrossRef]
- Raharjo, S.; Sofos, J.N.; Schmidt, G.R. Effect of Meat Curing Agents and Phosphates on Thiobarbituric Acid (TBA) Numbers of Ground Beef Determined by the Aqueous Acid Extraction TBA-C18 Method. Food Chem. 1992, 47, 137–143. [Google Scholar] [CrossRef]
- Wang, B.; Pace, R.D.; Dessai, A.P.; Bovell-Benjamin, A.; Phillips, B. Modified Extraction Method for Determining 2-Thiobarbituric Acid Values in Meat with Increased Specificity and Simplicity. J. Food Sci. 2002, 67, 2833–2836. [Google Scholar] [CrossRef]
- Wrona, M.; Silva, F.; Salafranca, J.; Nerín, C.; Alfonso, M.J.; Caballero, M.Á. Design of New Natural Antioxidant Active Packaging: Screening Flowsheet from Pure Essential Oils and Vegetable Oils to Ex Vivo Testing in Meat Samples. Food Control 2021, 120, 107536. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, W.; Yang, K.; Li, C. Effects of Sodium Alginate Edible Coating with Cinnamon Essential Oil Nanocapsules and Nisin on Quality and Shelf Life of Beef Slices during Refrigeration. J. Food Prot. 2022, 85, 896–905. [Google Scholar] [CrossRef]
- Hoa, V.B.; Song, D.H.; Seol, K.H.; Kang, S.M.; Kim, H.W.; Kim, J.H.; Cho, S.H. Coating with Chitosan Containing Lauric Acid (C12:0) Significantly Extends the Shelf-Life of Aerobically—Packaged Beef Steaks during Refrigerated Storage. Meat Sci. 2022, 184, 108696. [Google Scholar] [CrossRef]
- Stanojević-Nikolić, S.; Dimić, G.; Mojović, L.; Pejin, J.; Djukić-Vuković, A.; Kocić-Tanackov, S. Antimicrobial Activity of Lactic Acid Against Pathogen and Spoilage Microorganisms. J. Food Process. Preserv. 2016, 40, 990–998. [Google Scholar] [CrossRef]
- Reygaert, W.C. The Antimicrobial Possibilities of Green Tea. Front. Microbiol. 2014, 5, 434. [Google Scholar] [CrossRef]
- Ashrafi, A.; Jokar, M.; Mohammadi Nafchi, A. Preparation and Characterization of Biocomposite Film Based on Chitosan and Kombucha Tea as Active Food Packaging. Int. J. Biol. Macromol. 2018, 108, 444–454. [Google Scholar] [CrossRef]
- Demirok Soncu, E.; Arslan, B.; Ertürk, D.; Küçükkaya, S.; Özdemir, N.; Soyer, A. Microbiological, Physicochemical and Sensory Characteristics of Turkish Fermented Sausages (Sucuk) Coated with Chitosan-Essential Oils. LWT 2018, 97, 198–204. [Google Scholar] [CrossRef]
- Mojaddar Langroodi, A.; Nematollahi, A.; Sayadi, M. Chitosan Coating Incorporated with Grape Seed Extract and Origanum Vulgare Essential Oil: An Active Packaging for Turkey Meat Preservation. J. Food Meas. Charact. 2021, 15, 2790–2804. [Google Scholar] [CrossRef]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Ahmadi Gavlighi, H.; Gardini, F. Chitosan-Cinnamon Essential Oil Nano-Formulation: Application as a Novel Additive for Controlled Release and Shelf Life Extension of Beef Patties. Int. J. Biol. Macromol. 2017, 102, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Soares, N.; Silva, P.; Barbosa, C.; Pinheiro, R.; Vicente, A.A. Comparing the Effects of Glazing and Chitosan-Based Coating Applied on Frozen Salmon on Its Organoleptic and Physicochemical Characteristics over Six-Months Storage. J. Food Eng. 2017, 194, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Pematilleke, N.; Kaur, M.; Adhikari, B.; Torley, P.J. Relationship between Instrumental and Sensory Texture Profile of Beef Semitendinosus Muscles with Different Textures. J. Texture Stud. 2022, 53, 232–241. [Google Scholar] [CrossRef]
- Dong, C.; Wang, B.; Li, F.; Zhong, Q.; Xia, X.; Kong, B. Effects of Edible Chitosan Coating on Harbin Red Sausage Storage Stability at Room Temperature. Meat Sci. 2020, 159, 107919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, H.; Liu, J.; Zhang, H.; Huang, F. Changes in Eating Quality of Chinese Braised Beef Produced from Three Different Muscles. Int. J. Gastron. Food Sci. 2022, 29, 100584. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Karaman, S.; Dogan, M.; Yetim, H.; Kayacier, A. Characterization of O/W Model System Meat Emulsions Using Shear Creep and Creep Recovery Tests Based on Mechanical Simulation Models and Their Correlation with Texture Profile Analysis (TPA) Parameters. J. Food Eng. 2012, 108, 327–336. [Google Scholar] [CrossRef]
- Li, C.T. Myofibrillar Protein Extracts from Spent Hen Meat to Improve Whole Muscle Processed Meats. Meat Sci. 2006, 72, 581–583. [Google Scholar] [CrossRef]
- U-Chupaj, J.; Malila, Y.; Gamonpilas, C.; Kijroongrojana, K.; Petracci, M.; Benjakul, S.; Visessanguan, W. Differences in Textural Properties of Cooked Caponized and Broiler Chicken Breast Meat. Poult. Sci. 2017, 96, 2491–2500. [Google Scholar] [CrossRef]
- Paredes, J.; Cortizo-Lacalle, D.; Imaz, A.M.; Aldazabal, J.; Vila, M. Application of Texture Analysis Methods for the Characterization of Cultured Meat. Sci. Rep. 2022, 12, 3898. [Google Scholar] [CrossRef]
- Modzelewska-Kapituła, M.; Żmijewski, T. The Influence of Age and Gender on the Quality of Raw and Roasted Wild Boars (Sus Scrofa) Meat. Meat Sci. 2021, 181, 108600. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Colour Difference ∆E-A Survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Qin, Y.Y.; Yang, J.Y.; Lu, H.B.; Wang, S.S.; Yang, J.; Yang, X.C.; Chai, M.; Li, L.; Cao, J.X. Effect of Chitosan Film Incorporated with Tea Polyphenol on Quality and Shelf Life of Pork Meat Patties. Int. J. Biol. Macromol. 2013, 61, 312–316. [Google Scholar] [CrossRef]
- Gedarawatte, S.T.G.; Ravensdale, J.T.; Johns, M.L.; Li, M.; Al-Salami, H.; Dykes, G.A.; Coorey, R. Evaluation of the Water-Holding and Anti-Spoilage Effect of a Bacterial Cellulose Nanocrystal Coating for the Storage of Vacuum-Packaged Beef. Food Packag. Shelf Life 2022, 31, 100818. [Google Scholar] [CrossRef]
- Ramanathan, R.; Suman, S.P.; Faustman, C. Biomolecular Interactions Governing Fresh Meat Color in Post-Mortem Skeletal Muscle: A Review. J. Agric. Food Chem. 2020, 68, 12779–12787. [Google Scholar] [CrossRef]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How Muscle Structure and Composition Influence Meat and Flesh Quality. Sci. World J. 2016, 2016, 3182746. [Google Scholar] [CrossRef] [Green Version]
- Bekhit, A.E.A.; Morton, J.D.; Bhat, Z.F.; Kong, L. Meat Color: Factors Affecting Color Stability. Encycl. Food Chem. 2019, 2, 202–210. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total Volatile Basic Nitrogen (TVB-N) and Its Role in Meat Spoilage: A Review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Tamkutė, L.; Vaicekauskaitė, R.; Melero, B.; Jaime, I.; Rovira, J.; Venskutonis, P.R. Effects of Chokeberry Extract Isolated with Pressurized Ethanol from Defatted Pomace on Oxidative Stability, Quality and Sensory Characteristics of Pork Meat Products. LWT 2021, 150, 111943. [Google Scholar] [CrossRef]
- Sharma, H.; Mendiratta, S.K.; Agrawal, R.K.; Gurunathan, K.; Kumar, S.; Singh, T.P. Use of Various Essential Oils as Bio Preservatives and Their Effect on the Quality of Vacuum Packaged Fresh Chicken Sausages under Frozen Conditions. LWT 2017, 81, 118–127. [Google Scholar] [CrossRef]
- Cardoso, G.P.; Andrade, M.P.D.; Rodrigues, L.M.; Massingue, A.A.; Fontes, P.R.; de Lemos Souza Ramos, A.; Ramos, E.M. Retail Display of Beef Steaks Coated with Monolayer and Bilayer Chitosan-Gelatin Composites. Meat Sci. 2019, 152, 20–30. [Google Scholar] [CrossRef]
- Pabast, M.; Shariatifar, N.; Beikzadeh, S.; Jahed, G. Effects of Chitosan Coatings Incorporating with Free or Nano-Encapsulated Satureja Plant Essential Oil on Quality Characteristics of Lamb Meat. Food Control 2018, 91, 185–192. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Langroodi, A.M.; Molaei, R.; Mahmoudian, A. Chitosan-Starch Film Containing Pomegranate Peel Extract and Thymus Kotschyanus Essential Oil Can Prolong the Shelf Life of Beef. Meat Sci. 2020, 163, 108073. [Google Scholar] [CrossRef] [PubMed]
- Mariutti, L.R.B.; Bragagnolo, N. Influence of Salt on Lipid Oxidation in Meat and Seafood Products: A Review. Food Res. Int. 2017, 94, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Possamai, A.P.S.; Alcalde, C.R.; Feihrmann, A.C.; Possamai, A.C.S.; Rossi, R.M.; Lala, B.; Claudino-Silva, S.C.; Macedo, F.d.A.F. Shelf Life of Meat from Boer-Saanen Goats Fed Diets Supplemented with Vitamin E. Meat Sci. 2018, 139, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Chen, X.; Li, D. Chitosan Films and Coatings Containing Essential Oils: The Antioxidant and Antimicrobial Activity, and Application in Food Systems. Food Res. Int. 2016, 89, 117–128. [Google Scholar] [CrossRef]
- Vieira, J.M.; Mantovani, R.A.; Raposo, M.F.J.; Coimbra, M.A.; Vicente, A.A.; Cunha, R.L. Effect of Extraction Temperature on Rheological Behavior and Antioxidant Capacity of Flaxseed Gum. Carbohydr. Polym. 2019, 213, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Perumalla, A.V.S.; Hettiarachchy, N.S. Green Tea and Grape Seed Extracts—Potential Applications in Food Safety and Quality. Food Res. Int. 2011, 44, 827–839. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Kang, H.; Peng, X. Effect of Tannic Acid-Grafted Chitosan Coating on the Quality of Fresh Pork Slices during Cold Storage. Meat Sci. 2022, 188, 108779. [Google Scholar] [CrossRef]
- Shen, W.; Yan, M.; Wu, S.; Ge, X.; Liu, S.; Du, Y.; Zheng, Y.; Wu, L.; Zhang, Y.; Mao, Y. Chitosan Nanoparticles Embedded with Curcumin and Its Application in Pork Antioxidant Edible Coating. Int. J. Biol. Macromol. 2022, 204, 410–418. [Google Scholar] [CrossRef]
- Xiong, Y.; Kamboj, M.; Ajlouni, S.; Fang, Z. Incorporation of Salmon Bone Gelatine with Chitosan, Gallic Acid and Clove Oil as Edible Coating for the Cold Storage of Fresh Salmon Fillet. Food Control 2021, 125, 107994. [Google Scholar] [CrossRef]
- Karsli, B.; Caglak, E.; Prinyawiwatkul, W. Effect of High Molecular Weight Chitosan Coating on Quality and Shelf Life of Refrigerated Channel Catfish Fillets. LWT 2021, 142, 111034. [Google Scholar] [CrossRef]
- Kahve, H.I.; Duran, A. Evaluation of the Effect of Chitosan Coating on Microbiological and Oxidative Properties of Refrigerated Beef. Gida J. Food 2020, 45, 1154–1162. [Google Scholar] [CrossRef]
- Hamann, D.; Puton, B.M.S.; Comin, T.; Colet, R.; Valduga, E.; Zeni, J.; Steffens, J.; Junges, A.; Backes, G.T.; Cansian, R.L. Active Edible Films Based on Green Tea Extract and Gelatin for Coating of Fresh Sausage. Meat Sci. 2022, 194, 108966. [Google Scholar] [CrossRef]
- Song, X.C.; Canellas, E.; Wrona, M.; Becerril, R.; Nerin, C. Comparison of Two Antioxidant Packaging Based on Rosemary Oleoresin and Green Tea Extract Coated on Polyethylene Terephthalate for Extending the Shelf Life of Minced Pork Meat. Food Packag. Shelf Life 2020, 26, 100588. [Google Scholar] [CrossRef]







| Ch (% w/v) | Essential Oil (% w/v) |
|---|---|
| 0.5 (−1) a | 0.03 (−1) |
| 2 (1) | 0.03 (−1) |
| 0.5 (−1) | 0.1 (1) |
| 2 (1) | 0.1 (1) |
| 0.19 (−1.41) | 0.06 (0) |
| 2.31 (1.41) | 0.06 (0) |
| 1.25 (0) | 0.01 (−1.41) |
| 1.25 (0) | 0.12 (1.41) |
| 1.25 (0) | 0.06 (0) |
| 1.25 (0) | 0.06 (0) |
| 1.25 (0) | 0.06 (0) |
| Biopolymer | Concentration (%) | Essential Oil | Concentration (%) | pH | Contact Angle (°) |
|---|---|---|---|---|---|
| Chitosan | 0.5 | Flaxseed | 0.03 | 5.5 ± 0.1 | 16.9 ± 0.1 |
| 2 | 0.03 | 5.9 ± 0.1 | 19.6 ± 0.2 | ||
| 0.5 | 0.1 | 5.5 ± 0.1 | 17.1 ± 0.2 | ||
| 2 | 0.1 | 5.8 ± 0.1 | 22.3 ± 0.4 | ||
| 0.19 | 0.06 | 5.3 ± 0.0 | 14.9 ± 0.1 | ||
| 2.31 | 0.06 | 5.8 ± 0.1 | 23.7 ± 0.5 | ||
| 1.25 | 0.01 | 5.6 ± 0.1 | 17.9 ± 0.2 | ||
| 1.25 | 0.12 | 5.7 ± 0.1 | 17.1 ± 0.3 | ||
| 1.25 | 0.06 | 5.8 ± 0.0 | 19.1 ± 0.2 | ||
| 1.25 | 0.06 | 5.7 ± 0.1 | 18.9 ± 0.3 | ||
| 1.25 | 0.06 | 5.8 ± 0.0 | 19.0 ± 0.1 |
| Time (days) | Sample | Hardness (kg) | Adhesiveness (kg.s) | Resilience (%) | Cohesion | Springiness (%) | Gumminess | Chewiness |
|---|---|---|---|---|---|---|---|---|
| 0 | n/Ch–Sh | 10.93 ± 1.65 a | −94.54 ± 24.34 a | 13.89 ± 1.78 a | 0.21 ± 0.04 a | 34.60 ± 4.94 a | 2.31 ± 0.53 a | 0.81 ± 0.20 a |
| n/Ch–Kn | 12.10 ± 1.18 a | −79.97 ± 15.93 a | 12.82 ± 2.30 a | 0.21 ± 0.04 a | 26.97 ± 4.69 a | 2.56 ± 0.47 a | 0.70 ± 0.15 a | |
| n/Ch–St | 12.05 ± 1.01 a | −65.25 ± 17.01 a | 9.58 ± 1.00 a | 0.18 ± 0.04 a | 37.19 ± 5.09 a | 2.20 ± 0.35 a | 0.82 ± 0.16 a | |
| 5 | n/Ch–Sh | 12.31 ± 1.69 a | −108.50 ± 29.90 a | 13.07 ± 3.09 a | 0.22 ± 0.05 a | 37.64 ± 4.43 a | 2.60 ± 0.50 a | 0.91 ± 0.21 a |
| n/Ch–Kn | 13.24 ± 1.89 a | −88.50 ± 9.84 a | 10.25 ± 2.84 a | 0.19 ± 0.03 a | 35.94 ± 4.80 a | 2.61 ± 0.29 a | 0.93 ± 0.15 a | |
| n/Ch–St | 11.24 ± 2.32 a | −48.27 ± 3.79 a | 9.63 ± 3.40 a | 0.21 ± 0.06 a | 26.63 ± 4.51b | 2.26 ± 0.57 a | 0.61 ± 0.24 a,b | |
| Ch–Sh | 12.64 ± 2.06 a | −65.05 ± 20.41 a | 14.88 ± 2.63 a | 0.27 ± 0.06 a | 34.06 ± 5.09 a | 2.75 ± 0.53 a | 1.09 ± 0.12 a | |
| Ch–Kn | 12.06 ± 2.59 a | −61.75 ± 8.95 a | 11.39 ± 3.51 a | 0.20 ± 0.05 a | 27.14 ± 3.27 a | 2.33 ± 0.30 a | 0.62 ± 0.14 a | |
| Ch–St | 10.68 ± 1.94 a | −56.46 ± 5.47 a | 9.76 ± 1.38 a | 0.20 ± 0.04 a | 30.88 ± 2.71 a | 2.12 ± 0.33 a | 0.63 ± 0.08 a | |
| 8 | n/Ch–Sh | 9.92 ± 1.10 a | −122.90 ± 23.97 a | 15.72 ± 2.85 a | 0.24 ± 0.07 a | 46.26 ± 5.74 b | 2.42 ± 0.61 a | 1.35 ± 0.19 b |
| n/Ch–Kn | 11.27 ± 1.41 a | −122.95 ± 19.73 b | 14.47 ± 2.65 a | 0.21 ± 0.03 a | 34.34 ± 4.57 a | 2.40 ± 0.40 a | 0.92 ± 0.09 a | |
| n/Ch–St | 14.28 ± 1.25 a | −22.85 ± 2.74 b | 7.04 ± 0.52 b | 0.15 ± 0.02 a | 29.32 ± 0.96 b | 2.09 ± 0.11 a | 0.62 ± 0.05 b | |
| Ch–Sh | 11.65 ± 1.49 a | −67.03 ± 9.70 a | 13.95 ± 2.72 a | 0.24 ± 0.05 a | 38.65 ± 4.11 a | 2.80 ± 0.59 a | 1.12 ± 0.37 a | |
| Ch–Kn | 10.40 ± 0.94 a | −72.94 ± 17.54 a | 12.52 ± 2.97 a | 0.21 ± 0.05 a | 25.53 ± 3.87 a | 2.03 ± 0.53 a | 0.61 ± 0.14 a | |
| Ch–St | 11.27 ± 1.90 a | −49.92 ± 14.32 a | 10.79 ± 3.41 a | 0.22 ± 0.05 a | 33.60 ± 4.90 a | 2.33 ± 0.69 a | 0.77 ± 0.06 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, C.G.; Martins, J.T.; Lüdtke, F.L.; Geraldo, A.; Pereira, A.; Vicente, A.A.; Vieira, J.M. Chitosan Coating Functionalized with Flaxseed Oil and Green Tea Extract as a Bio-Based Solution for Beef Preservation. Foods 2023, 12, 1447. https://doi.org/10.3390/foods12071447
Mendes CG, Martins JT, Lüdtke FL, Geraldo A, Pereira A, Vicente AA, Vieira JM. Chitosan Coating Functionalized with Flaxseed Oil and Green Tea Extract as a Bio-Based Solution for Beef Preservation. Foods. 2023; 12(7):1447. https://doi.org/10.3390/foods12071447
Chicago/Turabian StyleMendes, Cíntia G., Joana T. Martins, Fernanda L. Lüdtke, Ana Geraldo, Alfredo Pereira, António A. Vicente, and Jorge M. Vieira. 2023. "Chitosan Coating Functionalized with Flaxseed Oil and Green Tea Extract as a Bio-Based Solution for Beef Preservation" Foods 12, no. 7: 1447. https://doi.org/10.3390/foods12071447