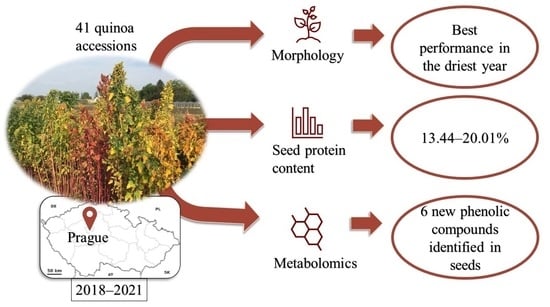
Nutritional Evaluation of Quinoa Genetic Resources Growing in the Climatic Conditions of Central Europe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Weather Conditions
2.3. Chemicals
2.4. Sample and Standard Preparation
2.5. UHPLC-ESI-MS/MS Instrumentation
2.6. UHPLC-ESI-MS/MS Analysis
2.7. Determination of the Phenolic Compound Concentration in Quinoa Samples
2.8. Chemical Analyses
2.9. Statistical Analyses
3. Results and Discussion
3.1. Weather Conditions
3.2. Morphological Evaluation
3.3. Crude Protein Content
3.4. Total Phenolic Content
3.5. Antioxidant Activity
3.6. Composition and Content of Phenolic Compounds
3.7. PCA Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fuentes, F.F.; Bazile, D.; Bhargava, A.; Martinez, E.A. Implications of farmers’ seed exchanges for on-farm conservation of quinoa, as revealed by its genetic diversity in Chile. J. Agric. Sci. 2012, 150, 702–716. [Google Scholar] [CrossRef] [Green Version]
- Bazile, D.; Jacobsen, S.E.; Verniau, A. The Global Expansion of Quinoa: Trends and Limits. Front. Plant Sci. 2016, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Razzaghi, F.; Jacobsen, S.E.; Jensen, C.R.; Andersen, M.N. Ionic and photosynthetic homeostasis in quinoa challenged by salinity and drought—Mechanisms of tolerance. Funct. Plant Biol. 2015, 42, 136–148. [Google Scholar] [CrossRef]
- Alandia, G.; Rodriguez, J.P.; Jacobsen, S.E.; Bazile, D.; Condori, B. Global expansion of quinoa and challenges for the Andean region. Glob. Food Secur.-Agric. Policy 2020, 26, 10. [Google Scholar] [CrossRef]
- FAO Climate Change and Food Security: Risks and Responses. Available online: https://www.fao.org/3/i5188e/I5188E.pdf (accessed on 15 January 2023).
- Angeli, V.; Silva, P.M.; Massuela, D.C.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium quinoa Willd.): An Overview of the Potentials of the “Golden Grain” and Socio-Economic and Environmental Aspects of Its Cultivation and Marketization. Foods 2020, 9, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niro, S.; D’Agostino, A.; Fratianni, A.; Cinquanta, L.; Panfili, G. Gluten-Free Alternative Grains: Nutritional Evaluation and Bioactive Compounds. Foods 2019, 8, 208. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Li, X.H.; Chen, P.X.; Zhang, B.; Liu, R.H.; Hernandez, M.; Draves, J.; Marcone, M.F.; Tsao, R. Assessing the fatty acid, carotenoid, and tocopherol compositions of amaranth and quinoa seeds grown in Ontario and their overall contribution to nutritional quality. J. Agric. Food Chem. 2016, 64, 1103–1110. [Google Scholar] [CrossRef]
- Maradini Filho, A.M.; Pirozi, M.R.; Borges, J.T.D.; Sant’Ana, H.M.P.; Chaves, J.B.P.; Coimbra, J. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar] [CrossRef]
- Craine, E.B.; Murphy, K.M. Seed composition and amino acid profiles for quinoa grown in Washington State. Front. Nutr. 2020, 7, 126. [Google Scholar] [CrossRef]
- Fan, X.; Guo, H.M.; Teng, C.; Zhang, B.; Blecker, C.; Ren, G.X. Anti-Colon Cancer Activity of Novel Peptides Isolated from In Vitro Digestion of Quinoa Protein in Caco-2 Cells. Foods 2022, 11, 194. [Google Scholar] [CrossRef]
- Shen, Y.B.; Zheng, L.Y.; Peng, Y.; Zhu, X.C.; Liu, F.; Yang, X.Q.; Li, H.M. Physicochemical, Antioxidant and Anticancer Characteristics of Seed Oil from Three Chenopodium quinoa Genotypes. Molecules 2022, 27, 2453. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, J.; Zou, L.; Fu, C.; Li, P.; Zhao, G. Chemical characterization, antioxidant, immune-regulating andanticancer activities of a novel bioactive polysaccharide from Chenopodium quinoa seeds. Int. J. Biol. Macromol. 2017, 99, 622–629. [Google Scholar] [CrossRef]
- Tan, M.H.; Zhao, Q.S.; Zhao, B. Physicochemical properties, structural characterization and biological activities of polysaccharides from quinoa (Chenopodium quinoa Willd.) seeds. Int. J. Biol. Macromol. 2021, 193, 1635–1644. [Google Scholar] [CrossRef]
- Dong, S.X.; Yang, X.S.; Zhao, L.; Zhang, F.X.; Hou, Z.H.; Xue, P. Antibacterial activity and mechanism of action saponins from Chenopodium quinoa Willd. husks against foodborne pathogenic bacteria. Ind. Crops Prod. 2020, 149, 112350. [Google Scholar] [CrossRef]
- Capraro, J.; De Benedetti, S.; Di Dio, M.; Bona, E.; Abate, A.; Corsetto, P.A.; Scarafoni, A. Characterization of Chenopodin Isoforms from Quinoa Seeds and Assessment of Their Potential Anti-Inflammatory Activity in Caco-2 Cells. Biomolecules 2020, 10, 795. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.J.; Zhu, K.L.; Yao, Y.; Chen, Y.H.; Guo, H.M.; Ren, G.X.; Yang, X.S.; Li, J.C. Antioxidant, anti-inflammatory, and antitumor activities of phenolic compounds from white, red, and black Chenopodium quinoa seed. Cereal Chem. 2020, 97, 703–713. [Google Scholar] [CrossRef]
- De Bock, P.; Van Bockstaele, F.; Muylle, H.; Quataert, P.; Vermeir, P.; Eeckhout, M.; Cnops, G. Yield and Nutritional Characterization of Thirteen Quinoa (Chenopodium quinoa Willd.) Varieties Grown in North-West Europe-Part I. Plants 2021, 10, 2689. [Google Scholar] [CrossRef]
- Matías, J.; Rodriguez, M.J.; Cruz, V.; Calvo, P.; Reguera, M. Heat stress lowers yields, alters nutrient uptake and changes seed quality in quinoa grown under Mediterranean field conditions. J. Agron. Crop Sci. 2021, 207, 481–491. [Google Scholar] [CrossRef]
- Garcia-Parra, M.; Roa-Acosta, D.; Garcia-Londono, V.; Moreno-Medina, B.; Bravo-Gomez, J. Structural Characterization and Antioxidant Capacity of Quinoa Cultivars Using Techniques of FT-MIR and UHPLC/ESI-Orbitrap MS Spectroscopy. Plants 2021, 10, 2159. [Google Scholar] [CrossRef]
- Granado-Rodriguez, S.; Aparicio, N.; Matias, J.; Perez-Romero, L.F.; Maestro, I.; Graces, I.; Pedroche, J.J.; Haros, C.M.; Fernandez-Garcia, N.; del Hierro, J.N.; et al. Studying the Impact of Different Field Environmental Conditions on Seed Quality of Quinoa: The Case of Three Different Years Changing Seed Nutritional Traits in Southern Europe. Front. Plant Sci. 2021, 12, 649132. [Google Scholar] [CrossRef] [PubMed]
- Janovska, D.; Jagr, M.; Svoboda, P.; Dvoracek, V.; Meglic, V.; Cepkova, P.H. Breeding Buckwheat for Nutritional Quality in the Czech Republic. Plants 2021, 10, 1262. [Google Scholar] [CrossRef]
- ČSN EN ISO 662 (588801); Živočišné a Rostlinné Tuky a Oleje—Stanovení Vlhkosti a Těkavých Látek. Ing. Jiří Hrazdil-Technické Normy: Brno, Czechia, 2001. (In Czech)
- ČSN EN ISO 20483 (46 1401); Obiloviny a Luštěniny—Stanovení Obsahu Dusíku a Výpočet Obsahu Dusíkatých Látek—Kjeldahlova Metoda. Technické Normy ČSN: Hradec Králové, Czech Republic, 2014; p. 24. (In Czech)
- Holasova, M.; Fiedlerova, V.; Smrcinova, H.; Orsak, M.; Lachman, J.; Vavreinova, S. Buckwheat—The source of antioxidant activity in functional foods. Food Res. Int. 2002, 35, 207–211. [Google Scholar] [CrossRef]
- Sensoy, I.; Rosen, R.T.; Ho, C.T.; Karwe, M.V. Effect of processing on buckwheat phenolics and antioxidant activity. Food Chem. 2006, 99, 388–393. [Google Scholar] [CrossRef]
- Cepkova, P.H.; Dostalikova, L.; Viehmannova, I.; Jagr, M.; Janovska, D. Diversity of quinoa genetic resources for sustainable production: A survey on nutritive characteristics as influenced by environmental conditions. Front. Sustain. Food Syst. 2022, 6, 960159. [Google Scholar] [CrossRef]
- Garcia-Parra, M.A.; Roa-Acosta, D.F.; Bravo-Gomez, J.E.; Hernandez-Criado, J.C.; Villada-Castillo, H.S. Effects of Altitudinal Gradient on Physicochemical and Rheological Potential of Quinoa Cultivars. Front. Sustain. Food Syst. 2022, 6, 862238. [Google Scholar] [CrossRef]
- Toubali, S.; Ait-El-Mokhtar, M.; Boutasknit, A.; Anli, M.; Ait-Rahou, Y.; Benaffari, W.; Ben-Ahmed, H.; Mitsui, T.; Baslam, M.; Meddich, A. Root Reinforcement Improved Performance, Productivity, and Grain Bioactive Quality of Field-Droughted Quinoa (Chenopodium quinoa). Front. Plant Sci. 2022, 13, 860484. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, Q.C.; Zhang, P.; Zhang, X.S.; Huang, T.Z.; Guo, Y.R.; Liu, J.N.; Li, L.; Li, H.X.; Qin, P. Transcriptomic and Metabolomic Analysis of the Response of Quinoa Seedlings to Low Temperatures. Biomolecules 2022, 12, 977. [Google Scholar] [CrossRef]
- Bioversity International; FAO. Descriptors for Quinoa (Chenopodium quinoa Willd.) and Wild Relatives; Bioversity International: Rome, Italy; FAO: Rome, Italy; Fundación PROINPA: Quillacollo, Bolivia; Instituto Nacional de Innovación Agropecuaria y Forestal: La Paz, Bolivia; International Fund for Agricultural Development: Rome, Italy, 2013; pp. 1–47. [Google Scholar]
- Maliro, M.F.A.; Guwela, V.F.; Nyaika, J.; Murphy, K.M. Preliminary Studies of the Performance of Quinoa (Chenopodium quinoa Willd.) Genotypes under Irrigated and Rainfed Conditions of Central Malawi. Front. Plant Sci. 2017, 8, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiam, E.; Allaoui, A.; Benlhabib, O. Quinoa Productivity and Stability Evaluation through Varietal and Environmental Interaction. Plants 2021, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, I.; Alseekh, S.; Shahid, M.; Leniak, E.; Wagner, M.; Mahmoudi, H.; Thushar, S.; Fernie, A.R.; Murphy, K.M.; Schmockel, S.M.; et al. The diversity of quinoa morphological traits and seed metabolic composition. Sci. Data 2022, 9, 323. [Google Scholar] [CrossRef]
- Manjarres-Hernandez, E.H.; Arias-Moreno, D.M.; Morillo-Coronado, A.C.; Ojeda-Perez, Z.Z.; Cardenas-Chaparro, A. Phenotypic Characterization of Quinoa (Chenopodium quinoa Willd.) for the Selection of Promising Materials for Breeding Programs. Plants 2021, 10, 1339. [Google Scholar] [CrossRef] [PubMed]
- Dumschott, K.; Wuyts, N.; Alfaro, C.; Castillo, D.; Fiorani, F.; Zurita-Silva, A. Morphological and Physiological Traits Associated with Yield under Reduced Irrigation in Chilean Coastal Lowland Quinoa. Plants 2022, 11, 323. [Google Scholar] [CrossRef]
- Benlhabib, O.; Boujartani, N.; Maughan, P.J.; Jacobsen, S.E.; Jellen, E.N. Elevated Genetic Diversity in an F2:6 Population of Quinoa (Chenopodium quinoa) Developed through an Inter-ecotype Cross. Front. Plant Sci. 2016, 7, 1222. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Wang, F.X.; Shock, C.C.; Meng, C.B.; Huang, Z.J.; Gao, L.; Zhao, J.Y. Evaluating quinoa stem lodging susceptibility by a mathematical model and the finite element method under different agronomic practices. Field Crops Res. 2021, 271, 108241. [Google Scholar] [CrossRef]
- Shah, S.S.; Shi, L.X.; Li, Z.J.; Ren, G.X.; Zhou, B.W.; Qin, P.Y. Yield, Agronomic and Forage Quality Traits of Different Quinoa (Chenopodium quinoa Willd.) Genotypes in Northeast China. Agronomy 2020, 10, 1908. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Wang, X.K.; Iqbal, S.; Hafeez, M.B.; Khan, S.; Raza, A.; Iqbal, J.; Maqbool, M.M.; Fiaz, S.; Qazi, M.A.; et al. Effect of Water Stress on Grain Yield and Physiological Characters of Quinoa Genotypes. Agronomy 2021, 11, 1934. [Google Scholar] [CrossRef]
- Prager, A.; Munz, S.; Nkebiwe, P.M.; Mast, B.; Graeff-Honninger, S. Yield and Quality Characteristics of Different Quinoa (Chenopodium quinoa Willd.) Cultivars Grown under Field Conditions in Southwestern Germany. Agronomy 2018, 8, 197. [Google Scholar] [CrossRef] [Green Version]
- Curti, R.N.; de la Vega, A.J.; Andrade, A.J.; Bramardi, S.J.; Bertero, H.D. Multi-environmental evaluation for grain yield and its physiological determinants of quinoa genotypes across Northwest Argentina. Field Crops Res. 2014, 166, 46–57. [Google Scholar] [CrossRef]
- Ahmadi, S.H.; Solgi, S.; Sepaskhah, A.R. Quinoa: A super or pseudo-super crop? Evidences from evapotranspiration, root growth, crop coefficients, and water productivity in a hot and semi-arid area under three planting densities. Agric. Water Manag. 2019, 225, 105784. [Google Scholar] [CrossRef]
- Pinto, A.A.; Fischer, S.; Wilckens, R.; Bustamante, L.; Berti, M.T. Production Efficiency and Total Protein Yield in Quinoa Grown under Water Stress. Agriculture 2021, 11, 1089. [Google Scholar] [CrossRef]
- Herzog, M.; Striker, G.G.; Colmer, T.D.; Pedersen, O. Mechanisms of waterlogging tolerance in wheat—A review of root and shoot physiology. Plant Cell Environ. 2016, 39, 1068–1086. [Google Scholar] [CrossRef] [PubMed]
- Arduini, I.; Baldanzi, M.; Pampana, S. Reduced Growth and Nitrogen Uptake during Waterlogging at Tillering Permanently Affect Yield Components in Late Sown Oats. Front. Plant Sci. 2019, 10, 1087. [Google Scholar] [CrossRef] [Green Version]
- Granado-Rodriguez, S.; Vilarino-Rodriguez, S.; Maestro-Gaitan, I.; Matias, J.; Rodriguez, M.J.; Calvo, P.; Cruz, V.; Bolanos, L.; Reguera, M. Genotype-Dependent Variation of Nutritional Quality-Related Traits in Quinoa Seeds. Plants 2021, 10, 2128. [Google Scholar] [CrossRef] [PubMed]
- Sobota, A.; Swieca, M.; Gesinski, K.; Wirkijowska, A.; Bochnak, J. Yellow-coated quinoa (Chenopodium quinoa Willd)—Physicochemical, nutritional, and antioxidant properties. J. Sci. Food Agric. 2020, 100, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- USDA. Agricultural Research Service. FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 30 July 2022).
- Schmidt, D.; Verruma-Bernardi, M.R.; Forti, V.A.; Borges, M. Quinoa and Amaranth as Functional Foods: A Review. Food Rev. Int. 2021, 37, 1–20. [Google Scholar] [CrossRef]
- Wang, N.; Wang, F.X.; Shock, C.C.; Meng, C.B.; Qiao, L.F. Effects of Management Practices on Quinoa Growth, Seed Yield, and Quality. Agronomy 2020, 10, 445. [Google Scholar] [CrossRef] [Green Version]
- Olgun, M.; Metin Kumlay, A.; Cemal Adiguzel, M.; Caglar, A. The effect of waterlogging in wheat (T. aestivum L.). Acta Agric. Scand. Sect. B–Soil Plant Sci. 2008, 58, 193–198. [Google Scholar]
- Stoychev, V.; Simova-Stoilova, L.; Vaseva, I.; Kostadinova, A.; Nenkova, R.; Feller, U.; Demirevska, K. Protein changes and proteolytic degradation in red and white clover plants subjected to waterlogging. Acta Physiol. Plant. 2013, 35, 1925–1932. [Google Scholar] [CrossRef]
- Wollmer, A.C.; Pitann, B.; Muhling, K.H. Grain storage protein concentration and composition of winter wheat (Triticum aestivurn L.) as affected by waterlogging events during stem elongation or ear emergence. J. Cereal Sci. 2018, 83, 9–15. [Google Scholar] [CrossRef]
- Hussain, M.A.; Naeem, A.; Sulieman, S.; Pitann, B.; Muhling, K.H. Sulfur uptake and distribution, grain yield, and quality of hybrid and inbred winter wheat (Triticum aestivum L.) varieties under early and late waterlogging. J. Plant Nutr. Soil Sci. 2022, 185, 622–631. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-Gonzales, R.; Ricci, A.; Fontecha, J.; Fernandez-Lopez, J.; Perez-Alvarez, J.A.; Viuda-Martos, M. Chemical, fatty acid, polyphenolic profile, techno-functional and antioxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd) seeds. Ind. Crops Prod. 2018, 111, 38–46. [Google Scholar] [CrossRef]
- Mhada, M.; Metougui, M.L.; El Hazzam, K.; El Kacimi, K.; Yasri, A. Variations of Saponins, Minerals and Total Phenolic Compounds Due to Processing and Cooking of Quinoa (Chenopodium quinoa Willd.) Seeds. Foods 2020, 9, 660. [Google Scholar] [CrossRef] [PubMed]
- Buitrago, D.; Buitrago-Villanueva, I.; Barbosa-Cornelio, R.; Coy-Barrera, E. Comparative Examination of Antioxidant Capacity and Fingerprinting of Unfractionated Extracts from Different Plant Parts of Quinoa (Chenopodium quinoa) Grown under Greenhouse Conditions. Antioxidants 2019, 8, 238. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.Y.; Yu, Q.Q.; Li, B.; Kan, J.Q. Comparative analysis of carotenoids and metabolite characteristics in discolored red pepper and normal red pepper based on non-targeted metabolomics. LWT-Food Sci. Technol. 2022, 153, 11. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B.S. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samec, D.; Karalija, E.; Sola, I.; Bok, V.V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Reguera, M.; Conesa, C.M.; Gil-Gomez, A.; Haros, C.M.; Perez-Casa, M.A.; Briones-Labarca, V.; Bolanosi, L.; Bonilla, I.; Alvarez, R.; Pinto, K.; et al. The impact of different agroecological conditions on the nutritional composition of quinoa seeds. PeerJ 2018, 6, e4442. [Google Scholar] [CrossRef]
- Antognoni, F.; Potente, G.; Biondi, S.; Mandrioli, R.; Marincich, L.; Ruiz, K.B. Free and Conjugated Phenolic Profiles and Antioxidant Activity in Quinoa Seeds and Their Relationship with Genotype and Environment. Plants 2021, 10, 1046. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.H.; Zhang, B.; Chen, P.X.; Liu, R.H.; Tsao, R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 166, 380–388. [Google Scholar] [CrossRef]
- Nowak, V.; Du, J.; Charrondiere, U.R. Assessment of the nutritional composition of quinoa (Chenopodium quinoa Willd.). Food Chem. 2016, 193, 47–54. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, B.; Li, X.H.; Chen, P.X.; Zhang, H.; Liu, R.H.; Tsao, R. Bound Phenolics of Quinoa Seeds Released by Acid, Alkaline, and Enzymatic Treatments and Their Antioxidant and alpha-Glucosidase and Pancreatic Lipase Inhibitory Effects. J. Agric. Food Chem. 2016, 64, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Shkondrov, A.M.; Krasteva, I.N. High Resolution LC-MS/MS Screening for secondary Metabolites in Bulgarian Species of Genus Astragalus L. Química Nova 2021, 44, 683–688. [Google Scholar] [CrossRef]
- Vasilev, H.; Smejkal, K.; Gronover, C.S.; Choi, Y.H.; Prufer, D.; Jankovska, D.; Ionkova, I. Flavonol glycosides from aerial parts of Astragalus thracicus Griseb. Phytochem. Lett. 2021, 41, 119–122. [Google Scholar] [CrossRef]
- Dini, I.; Tenore, G.C.; Dini, A. Nutritional and antinutritional composition of Kancolla seeds: An interesting and underexploited andine food plant. Food Chem. 2005, 92, 125–132. [Google Scholar] [CrossRef]
- Gomez-Caravaca, A.M.; Iafelice, G.; Lavini, A.; Pulvento, C.; Caboni, M.F.; Marconi, E. Phenolic Compounds and Saponins in Quinoa Samples (Chenopodium quinoa Willd.) Grown under Different Saline and Nonsaline Irrigation Regimens. J. Agric. Food Chem. 2012, 60, 4620–4627. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, M.F.; Vieira, I.J.C.; Sant’Anna, C.M.R.; da Silva, D.R.; Maia, A.I.V.; Braz-Filho, R.; Vieira-da-Motta, O.; Mathias, L. New triterpene glycoside and other chemical constituents from the leaves of Swartzia apetala Raddi var. glabra. Nat. Prod. Res. 2013, 27, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Kicel, A.; Wolbis, M. Study on the phenolic constituents of the flowers and leaves of Trifolium repens L. Nat. Prod. Res. 2012, 26, 2050–2054. [Google Scholar] [CrossRef]
- Popovic, Z.; Matic, R.; Bajic-Ljubicic, J.; Tesevic, V.; Bojovic, S. Geographic variability of selected phenolic compounds in fresh berries of two Cornus species. Trees-Struct. Funct. 2018, 32, 203–214. [Google Scholar] [CrossRef]
- Richane, A.; Rim, B.; Wided, M.; Riadh, K.; Khaoula, A.; Nizar, M.; Hanen, B. Variability of phenolic compounds and antioxidant activities of ten Ceratonia siliqua L. provenances. Biochem. Syst. Ecol. 2022, 104, 104486. [Google Scholar] [CrossRef]
- Ismail, H.; Maksimovic, J.D.; Maksimovic, V.; Shabala, L.; Zivanovic, B.D.; Tian, Y.; Jacobsen, S.E.; Shabala, S. Rutin, a flavonoid with antioxidant activity, improves plant salinity tolerance by regulating K+ retention and Na+ exclusion from leaf mesophyll in quinoa and broad beans. Funct. Plant Biol. 2016, 43, 75–86. [Google Scholar] [CrossRef]
- Ma, D.Y.; Sun, D.X.; Wang, C.Y.; Li, Y.G.; Guo, T.C. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Galieni, A.; Di Mattia, C.; De Gregorio, M.; Speca, S.; Mastrocola, D.; Pisante, M.; Stagnari, F. Effects of nutrient deficiency and abiotic environmental stresses on yield, phenolic compounds and antiradical activity in lettuce (Lactuca sativa L.). Sci. Hortic. 2015, 187, 93–101. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Ind. Crops Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Jiang, F.; Ren, Y.J.; Du, C.W.; Nie, G.; Liang, J.B.; Yu, X.Z.; Du, S.K. Effect of pearling on the physicochemical properties and antioxidant capacity of quinoa (Chenopodium quinoa Willd.) flour. J. Cereal Sci. 2021, 102, 103330. [Google Scholar] [CrossRef]
- Stikic, R.I.; Milincic, D.D.; Kostic, A.Z.; Jovanovic, Z.B.; Gasic, U.M.; Tesic, Z.L.; Djordjevic, N.Z.; Savic, S.K.; Czekus, B.G.; Pesic, M.B. Polyphenolic profiles, antioxidant, and in vitro anticancer activities of the seeds of Puno and Titicaca quinoa cultivars. Cereal Chem. 2020, 97, 626–633. [Google Scholar] [CrossRef]
- Rossi, L.; Borghi, M.; Francini, A.; Lin, X.L.; Xie, D.Y.; Sebastiani, L. Salt stress induces differential regulation of the phenylpropanoid pathway in Olea europaea cultivars Frantoio (salt-tolerant) and Leccino (salt-sensitive). J. Plant Physiol. 2016, 204, 8–15. [Google Scholar] [CrossRef]
- Zafari, S.; Sharifi, M.; Chashmi, N.A.; Mur, L.A.J. Modulation of Pb-induced stress in Prosopis shoots through an interconnected network of signaling molecules, phenolic compounds and amino acids. Plant Physiol. Biochem. 2016, 99, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.Q.; Charles, M.T.; Luo, Z.S.; Mimee, B.; Veronneau, P.Y.; Rolland, D.; Roussel, D. Preharvest Ultraviolet C Irradiation Increased the Level of Polyphenol Accumulation and Flavonoid Pathway Gene Expression in Strawberry Fruit. J. Agric. Food Chem. 2017, 65, 9970–9979. [Google Scholar] [CrossRef]
- Kim, J.H.; Duan, S.; Lim, Y.J.; Eom, S.H. Changes in Quercetin Derivatives and Antioxidant Activity in Marigold Petals (Tagetes patula L.) Induced by Ultraviolet-B Irradiation and Methyl Jasmonate. Plants 2022, 11, 2947. [Google Scholar] [CrossRef] [PubMed]
- Ancillotti, C.; Bogani, P.; Biricolti, S.; Calistri, E.; Checchini, L.; Ciofi, L.; Gonnelli, C.; Del Bubba, M. Changes in polyphenol and sugar concentrations in wild type and genetically modified Nicotiana langsdorffii Weinmann in response to water and heat stress. Plant Physiol. Biochem. 2015, 97, 52–61. [Google Scholar] [CrossRef]
- Wang, J.Y.; Yuan, B.; Huang, B.R. Differential Heat-Induced Changes in Phenolic Acids Associated with Genotypic Variations in Heat Tolerance for Hard Fescue. Crop Sci. 2019, 59, 667–674. [Google Scholar] [CrossRef]
- Mohammadi, H.; Rahimpour, B.; Pirasteh-Anosheh, H.; Race, M. Salicylic Acid Manipulates Ion Accumulation and Distribution in Favor of Salinity Tolerance in Chenopodium quinoa. Int. J. Environ. Res. Public Health 2022, 19, 1576. [Google Scholar] [CrossRef]
- Mariotti, L.; Reyes, T.H.; Ramos-Diaz, J.M.; Jouppila, K.; Guglielminetti, L. Hormonal Regulation in Different Varieties of Chenopodium quinoa Willd. Exposed to Short Acute UV-B Irradiation. Plants 2021, 10, 858. [Google Scholar] [CrossRef] [PubMed]
- Pasko, P.; Sajewicz, M.; Gorinstein, S.; Zachwieja, Z. Analysis of Selected Phenolic Acids and Flavonoids in Amaranthus cruentus and Chenopodium quinoa Seeds and Sprouts by HPLC. Acta Chromatogr. 2008, 20, 661–672. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Caravaca, A.M.; Iafelice, G.; Verardo, V.; Marconi, E.; Caboni, M.F. Influence of pearling process on phenolic and saponin content in quinoa (Chenopodium quinoa Willd.). Food Chem. 2014, 157, 174–178. [Google Scholar] [CrossRef]
- Ma, A.M.; Qi, X.Q. Mining plant metabolomes: Methods applications, and perspectives. Plant Commun. 2021, 2, 100238. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.T.; Wu, Z.R.; Xi, L.L.; Li, X.; Chen, P.; Wang, F.Q.; Meng, W.B.; Zhou, W.C.; Wu, X.A.; Yao, X.J.; et al. Inhibition of invasion by N-trans-feruloyloctopamine via AKT, p38MAPK O crossMark and EMT related signals in hepatocellular carcinoma cells. Bioorg. Med. Chem. Lett. 2017, 27, 989–993. [Google Scholar] [CrossRef]
- Lim, E.J.; Kang, H.J.; Jung, H.J.; Kim, K.; Lim, C.J.; Park, E.H. Anti-inflammatory, Anti-Angiogenic and Anti-Nociceptive Activities of 4-Hydroxybenzaldehyde. Biomol. Ther. 2008, 16, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Kim, D.S.; Kang, S. Gastrodia elata Blume water extracts improve insulin resistance by decreasing body fat in diet-induced obese rats: Vanillin and 4-hydroxybenzaldehyde are the bioactive candidates. Eur. J. Nutr. 2011, 50, 107–118. [Google Scholar] [CrossRef]
- Xi, K.Y.; Xiong, S.J.; Li, G.; Guo, C.Q.; Zhou, J.; Ma, J.W.; Yin, J.L.; Liu, Y.Q.; Zhu, Y.X. Antifungal Activity of Ginger Rhizome Extract against Fusarium solani. Horticulturae 2022, 8, 983. [Google Scholar] [CrossRef]
- Janeczko, M.; Maslyk, M.; Kubinski, K.; Golczyk, H. Emodin, a natural inhibitor of protein kinase CK2, suppresses growth, hyphal development, and biofilm formation of Candida albicans. Yeast 2017, 34, 253–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verebova, V.; Benes, J.; Stanicova, J. Biophysical Characterization and Anticancer Activities of Photosensitive Phytoanthraquinones Represented by Hypericin and Its Model Compounds. Molecules 2020, 25, 5666. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Chung, J.G. Anticancer potential of emodin. Biomedicine 2012, 2, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, S.; Preziuso, F.; Epifano, F.; Scotti, L.; Bucciarelli, T.; Taddeo, V.A.; Genovese, S. Novel biologically active principles from spinach, goji and quinoa. Food Chem. 2019, 276, 262–265. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dostalíková, L.; Hlásná Čepková, P.; Janovská, D.; Svoboda, P.; Jágr, M.; Dvořáček, V.; Viehmannová, I. Nutritional Evaluation of Quinoa Genetic Resources Growing in the Climatic Conditions of Central Europe. Foods 2023, 12, 1440. https://doi.org/10.3390/foods12071440
Dostalíková L, Hlásná Čepková P, Janovská D, Svoboda P, Jágr M, Dvořáček V, Viehmannová I. Nutritional Evaluation of Quinoa Genetic Resources Growing in the Climatic Conditions of Central Europe. Foods. 2023; 12(7):1440. https://doi.org/10.3390/foods12071440
Chicago/Turabian StyleDostalíková, Lucie, Petra Hlásná Čepková, Dagmar Janovská, Pavel Svoboda, Michal Jágr, Václav Dvořáček, and Iva Viehmannová. 2023. "Nutritional Evaluation of Quinoa Genetic Resources Growing in the Climatic Conditions of Central Europe" Foods 12, no. 7: 1440. https://doi.org/10.3390/foods12071440