3.2.1. Physicochemical Characteristics of the Functional Breads
Colour, moisture, ash, and the protein distribution of breads enriched with BSGs are reported in
Table 5 and compared with the characteristics of the control bread.
Regarding colour indices, the crust of the control bread showed the highest L* (together with Em-D breads) and b* (with Da-S-20G3) values and the lowest a* indices (with Da-D-25G4, Da-S-20G3, and Em-D-20G3). Em-S-25G4 had the lowest L* and b* values, while the highest a* was measured on Da-S-25G4. The crumb of the control bread also showed the highest L* and b* (with Ri-D-20G3) values and the lowest a* indices.
The type of BSG exerted significant effects on all colour indices. Concerning crust, the highest brightness and yellow index were measured on Da samples and the lowest red index was detected in Ri breads. The crumb of Ri breads was the darkest, the reddest (with Da samples), and showed the lowest yellow index (together with Em). The different behaviour observed for the various types of BSG was correlated to the colour of the starting spent grains.
The level of BSG-gluten addition significantly affected the colorimetric indices of bread with the exception of the crust a* values, but the effects were opposite between crust and crumb. The increase in BSG-gluten content generally decreased the L* and b* values of the crust, i.e., determined the formation of a darker colour. This behaviour is explained by the higher protein content in the formulation which caused intensive Maillard reactions [
53]. Contrary to what happened to the crust, the increase in BSG-gluten content corresponded to a slightly lighter crumb (due to the clear colour of the fibre added) but also to a more intense golden colour (increased a* and b* values). In most previous works, a darker crumb was observed as a consequence of BSG addition [
15] but Gómez et al. [
54] pointed out that the effects depend on the colour of fibres contained in the added ingredient.
Remarkable water content was quantified in the control bread. However, other bread samples showed higher crust moisture (Em-D-20G3) and similar amounts of water in crumb (Em-D-25G4). The type of BSG did not affect crust moisture while the crumb of breads produced with Da spent grains contained generally higher water in their composition. The crumb moisture was significantly and positively affected by the BSG-gluten level, and this behaviour may be attributed to the higher fibre and protein contribution [
17].
Regarding ash, the lowest and the highest amounts were quantified in control (3.1%) and Em-S-25G4 (3.6%) breads, respectively. The addition of all the BSG types significantly increased bread ash content but, consistently with the BSG composition reported in
Table 3, the greatest ash amount was contributed by the Em type. Since BSG is mainly composed of the husk of grain and minerals are present in a greater amount in their outer layer, the amount of BSG-gluten added also had a significant and positive effect on the bread ash content [
17,
55].
Concerning protein size distribution in bread samples, the chromatographic profiles highlighted the presence of only two main peaks, eluted between 10 and 12.5 min (i.e., the range of large and small monomeric proteins) and the total lack of peaks in the elution range of polymeric protein aggregates. This result could be due to a variety of factors including technological process; BSG diluting effect on the protein network; and possible accumulation of low-molecular weight metabolites (mainly glutathione) deriving from the lysed yeast cell. Glutathione (GSH) was found to be responsible for the chemical modification of the gluten protein structure, leading to its depolymerization [
56] and the formation of lower molecular weight gluten proteins [
57,
58]. Consequently to these changes, unextractable proteins included only monomeric forms (
Table 5). From the comparison of data in
Table 3 and
Table 5, a remarkable increase in UP% from BSGs to the corresponding breads can be inferred. It could be due to baking, whose high temperatures are known to induce interactions among different class of proteins, thus leading to the formation of larger aggregates or a supramolecular structure. The average unextractable monomeric proteins ranged from a minimum of 35.91% in Em-S-20G3 to a maximum of 42.07% in Ri-S-20G3. Percentages higher than 40% were also detected in Ri-D-25G4, Ri-S-25G4, and the control bread. Significant single effects of BSG type were observed, with UP% higher in Ri breads and lower in Em breads.
The geographical origin of the starting cereal mixtures did not significantly influence any of the parameters of
Table 5.
3.2.2. Phenolic Concentration, Antioxidant Activity, and Fibre Content of the Functional Breads
The amounts of antioxidants and fibres are among the most important factors to consider in assessing the nutraceutical quality of breads.
The total phenolic contents of functional breads were always higher than those of control bread (1.555 ± 0.158 mg/g d.m.) and comprised between 1.793 ± 0.183 mg/g d.m. (Ri-D-20G3) and 2.833 ± 0.772 mg/g d.m. (Da-S-25G4) (
Table 6). These were the results of the interactions among the type of BSG, the geographical origin of cereal mixtures, and the amount of BSG-gluten, although the single effects of these variables were also statistically significant (with the exception of the geographical origin). In particular, the highest TPCs were detected in Em breads, consistently with the high phenolic concentrations of the starting spent grains. Moreover, the bread antioxidant content increased with the increase in BSG-gluten amount in the bread formulation. Finally, it is appropriate to consider that the phenolic content of the final breads was also affected by bread-making and, although the same process was applied for all samples, the magnitude of these changes could not be the same. According to a recent research of Tian et al. [
59], bread-making generally improved the potential health benefits of whole wheat products. The authors pointed out that fermentation and baking generally increased soluble phenolic content and its antioxidant activity due to the contribution of Maillard reaction products, and that those steps only slightly increased the insoluble phenolic fraction and its antioxidant activity. The TPCs of our experimental functional breads were considerable higher than those (0.47 ± 0.06 mg/g d.m.) retrieved in the recent literature [
60].
The antioxidant activity values of the functional breads showed trends similar to those of TPCs. They were always higher than those of the control bread (0.36 ± 0.02 mg Trolox/g d.m.) and comprised between 0.67 ± 0.16 mg/g d.m. (Ri-D-20G3) and 3.45 ± 0.47 mg/g d.m. (Em-D-25G4) (
Table 6). The single effects of the type of BSG and the amount of BSG-gluten were statistically significant. In particular, the highest and the lowest antioxidant activity values were detected in breads produced with Em and Ri spent grains, respectively. Furthermore, the bread antioxidant activity increased with the increase in BSG-gluten amount in the bread formulation. TPC and antioxidant activity showed a strong correlation (R = 0.885,
p-value < 0.01).
The bread phenolic profiles were simpler than those of the starting BSGs. Five phenolic compounds were identified in all the functional breads, including four phenolic acids (gallic, vanillic, caffeic, and sinapic) and a flavanol (epicagallotechin gallate). Nevertheless, their concentrations depended on BSG type, the geographical origin of the stating cereal mixtures, and BSG-gluten amount. p-Coumaric acid was detected only in the control bread that, instead, did not contain phenolics such as epigallocatechin gallate, and vanillic, caffeic, and sinapic acids. The interactive effects of the three factors were also statistically significant. The type of BSG showed significant single effects, with the highest concentrations of almost all compounds detected in Em breads. The higher level of BSG-gluten supplementation allowed to obtain breads with a higher content of almost all compounds. The single effects of the geographical origin of the starting mixtures were less significant.
The interest towards the fibre content of BSG-enriched bread is due to the possibility, established by Reg. (EU) N°. 432/2012 [
61], to use the following two health claims: “Barley grain fibre contributes to an increase in faecal bulk” for foods which are high in that fibre, i.e., for those foods that contain at least 6 g of fibre per 100 g or at least 3 g of fibre per 100 kcal; and “Beta-glucans contribute to the maintenance of normal blood cholesterol levels” for food which contains at least 1 g of beta-glucans from barley/barley bran per quantified portion. [
62]. The increase of faecal bulk is related to the ingestion of insoluble dietary fibre. Other long-known benefits of dietary fibre intake include the modulation of glycaemic index and potential prebiotic capacity, which are known to be linked to arabinoxylan and arabinoxylan-oligosaccharides, the latter primarily deriving from wheat [
63,
64]. USDA recommends daily intakes of fibres equal to 25 g for women and 38 g for men up to 50 years old and to 21 and 30 g for elder women and men, respectively [
65]. Since the actual intake is generally lower, especially in Western countries, the regular consumption of BSG-enriched breads could help consumers to meet such recommendations. As can be inferred from
Table 6, the amounts of IDF and SDF in BSG- enriched breads were significantly lower than in the corresponding spent grains, due to the dilution effect of Manitoba flour. IDF% and SDF% felt both interactive and single effects of the factors. More in depth, IDF% ranged from 2.05% in the control bread to 7.15% of Em-S-25G4 while SDF% was in the 0.004% (Ri-S-25G4)–1.56% (Da-S-25G4) range and the percentages of soluble fibre on the total fibre were appreciably higher than in the BSG samples, ranging from 0.06% (RiS25-G4) to 22.51% (DaS25-G4), thus contributing beneficial effects that go beyond the simple increase in faecal bulk. A first reason for this behaviour is the high percentages of soluble fibre in the Manitoba flour. In fact, the control bread had a percentage of soluble fibre on the total fibres equal to 23.49%. Another reason is that, during bread-making, a decomposition of dietary fibre (first hemicellulose and afterwards cellulose) occurred, reducing the fibre molecular weight [
66]. The reason for this degrading action could be both the yeast, since some
Saccharomyces cerevisiae strains are able to produce cellulase and xylanase [
66], and the first step of the baking process when both temperatures and moisture are elevated, thus simulating the conditions of an autoclave treatment [
67]. In the control bread, the incidence of soluble fibre on the total amount of fibre was equal to 23.49%. However, while data indicate that the single effects exerted by BSG type and the geographical origin on bread IDF% were similar to those observed for spent grains, they also describe the opposite effects of BSG type and geographical origin on the soluble fibre fraction between breads and spent grains. Significant increases in both IDF% and SDF% were evaluated by increasing the amount of BSG-gluten added. The total dietary fibres ranged between 4.50% of RiD20-G3 and 7.85% of EmS25-G4, resulting as significantly higher than those quantified in the control bread (2.68%) and slightly higher than the ranges found in recent literature (3.32–6.37%) [
44]. According to the data concerning the dietary fibre contents of the enriched breads, the inclusion of Em spent grains in percentages equal or higher than those used in these experiments could allow the use of one or both of the health claims mentioned above [
61].
3.2.3. Structural Characteristics of the Functional Breads
Consumers are more likely to purchase well-leavened and regularly shaped breads. For this reason, height and specific volume are often considered as the key quality parameters.
In our work, the minimum and maximum slice heights were used as indices of bread shape regularity. The highest values of the minimum (9.36 ± 0.24 cm) and maximum (10.14 ± 0.17 cm) height were measured on the control bread, while the lowest values were found for Da-S-20G3 (7.72 ± 0.84 cm) and Ri-D-25G4 (8.39 ± 0.20 cm), respectively (
Table 7). As can be inferred from these data, slice height values were significantly affected by interactions among BSG type, origin, and the amount of BSG-gluten, but the BSG type also exerted significant single effects, with the highest values observed in Em breads. The crumb specific volume is another key parameter since superior bread quality is often characterised by high specific volumes [
44]. Crumb specific volume (
Table 7) was only slightly reduced by the BSG addition, ranging from 2.11 ± 0.9 cm
3/g of Da-D-20G3 to 2.64 cm
3/g of Em-D-25G4 and control bread. These results were significantly better than those observed by Amoriello et al. [
15] in breads produced with medium or strong wheat flour supplemented with 5 or 10% of BSG. In that work, the authors attributed the limited dough development of BSG-enriched breads to a reduction of extensibility and the gas-retention ability of gluten, in turn caused by dilution with non-gluten proteins and disruption due to the interference of fibres. The better specific volume of supplemented breads obtained in our work was determined by the concurrent addition of gluten. As always in our work, only the interactive effects of the three factors were significant, but it is interesting to point out that, although not significant, the highest specific volumes were quantified in Em breads, especially at increasing amounts of BSG-gluten added, i.e., in the samples with the highest fibre contents and deriving from the spent grains that had the highest amounts of total and large unextractable polymeric proteins.
Pores are created within the dough structure as a consequence of CO
2 production during leavening. Their characteristics are described in
Table 7. The control bread had a high pore density (0.73 ± 0.02 pores/cm
2) but not the highest, since that index ranged from ~0.3 pores/cm
2 (Ri-S breads, Em-D-25G4, and Em-S-25G4) to ~0.82 pores/cm
2 (Em-D-20G3). This result was not in agreement with the findings of Neylon et al. [
44], who observed a significant decrease in the number of cells in BSG-fortified breads. BSG type, origin, and the amount of BSG-gluten exerted statistically significant single and interactive effects, with the higher density measured on breads produced with Da-D spent grains at the lowest level of addition. The average pore size was inversely correlated (R = 0.938,
p-value < 0.01) with pore density, ranging from 0.10 mm
2 (control bread and Da-S-25G4) to 0.30 mm
2 (Em-D-25G4 and Ri-S-20G3). As for pore density, the results were not in agreement with the findings of Neylon et al. [
44], who observed a decrease in the cell diameter in BSG-fortified breads. Only the BSG type showed a significant effect on pore size, with the lowest values observed in Da breads. Porosity % ranged from ~35% (control bread, Da-D-20G3, and Ri-D-25G4) to ~47% (Em-S-25G4) and, as for the average pore size, only the BSG type showed a significant effect, with the highest values observed in Em breads. According to these results, the crumb of Em breads had a less compact structure than those of all other breads. Porosity % was well correlated with the total pore surface (pore density × verage pore size), showing an R value of 0.694 (
p-value < 0.01). Circularity ranged from 0.774 ± 0.029 (Ri-S-20G3) to 0.829 ± 0.029 (Em-S-25G4), thus indicating a predominantly circular shape. Nevertheless, data showed a remarkable variability already within the samples of each type of bread and only the interactive effects of the three factors were statistically significant, making it difficult to understand the weight of each independent variable.
3.2.4. Sensorial Characteristics of the Functional Breads
Control bread and breads enriched with the BSGs were evaluated by a trained panel through a Quantitative Descriptive Analysis and the results are reported in
Table 8. Two of the characteristics that judges were requested to evaluate, namely crust bitterness and crumb stickiness, were not detected for any of the experimental breads. The interactive effects of BSG type, the origin of cereal mixtures, and the amount of BSG-gluten added were significant for all the other sensorial parameters.
Bread colour is the first variable evaluated by consumers and strongly affects their willingness to purchase and the product acceptability. In agreement with the findings of Ginindza et al. [
68], the addition of BSG always resulted in a significant colour change compared with the control bread and the reason is that BSG contributed a higher amount of aminoacids, thus favouring the non-enzymatic browning reactions [
69]. The Em breads were evaluated as the darkest. The single effects of geographical origin and the amount of BSG-gluten added were not significant either for crust or for crumb colour. Regarding the other visual characteristics, the type of BSG had significant single effects on crust thickness (higher in Da breads while control bread had intermediate scores), crumb pore size (higher in Ri breads while control bread had intermediate scores), and crumb development (higher in Em samples among the BSG-enriched breads but lower than that of the control bread).
The fortified breads generally showed the highest intensity of wheat, malt, and yeast flavours. According to Ktenioudaki et al. [
16], these flavours were due to volatile compounds already present in BSG (2-heptane, butanal, 2-methylbutanale, benzene, and 2,3-butanedione) and arising from fermentation (ethanol, butanol, and other acholic compounds) and Maillard reactions (furfural, pyrazine). The type of BSG exerted significant effects on overall intensity, malty, and toasty flavours (lower in Em breads), and on wheat and yeast smells (higher in Da breads). The amount of BSG-gluten added exerted significant single effects only on wheat and yeast smells, which increased with the level of replacement.
Concerning crumb taste, the single effects of the three factors were not statistically significant. The saltiness and acidity/sourness of the functional breads were evaluated as lower or equal to those of the control bread. The latter obtained intermediate scores for sweetness, perhaps as a consequence of the maltose and glucose contained in BSG. Moreover, the sweet taste was also enhanced by the volatile compounds responsible for sweet and malty flavours.
Regarding bread texture, the addition of BSG-gluten always increased resistance to chewing and the fibrousness of the functional breads with respect to the control. The control bread obtained intermediate scores for crispness. The highest crust hardness and resistance to chewing and the lowest cohesiveness and fibrousness were attributed to breads fortified with Da spent grains. The amount of BSG-gluten added significantly affected the bread texture, except for crumb cohesiveness. The hardness of the crust and the resistance to chewing of crumb increased with BSG-gluten content. This behaviour could be explained by both the higher fibre and protein contents of functional breads, which caused a greater water absorption [
17,
70], and also the presence of pentopans, a fibre BSG component, responsible for the gluten protein cross linking [
71]. Crust crispness decreased with the increase in BSG-gluten content due to the increase in water absorbed, while crumb cohesiveness and fibrousness did not show significant changes. These results were only partially in agreement with those of Yitayew et al. [
17], who found that the hardness and breakage of crumb increased with BSG level.
Finally, the best and the worse overall ratings were attributed to Ri (together with control) and Em breads, respectively, and the panellists accorded their preferences to breads produced with the lowest amount of BSG-gluten added only for DaS and Em breads. In the other case, the % of BSG added did not affect the overall sensory quality. This is an interesting finding since the percentages of BSG in our products (20 or 25%) was much higher than those (≤10%) indicated as optimal by Yitayew et al. [
17] and Ginindza et al. [
68] who found the maximum and minimum acceptability score for the control sample and the bread supplemented with the highest amount of BSG, respectively. The interest of our finding is also due to the fact that, in other research, the BSG-fortified foods exerted a higher appeal [
42] than the not-enriched counterparts as a consequence of consumer interest for health issues, characteristics that overshadows the product sensory properties [
72].
3.2.5. Principal Component Analysis and Pearson Coefficients
Principal Component Analysis was carried out to verify the possibility of grouping BSGs by type and/or by place of origin on the basis of their physical characteristics and the content of antioxidants, fibres, and non-extractable proteins. The ability of PCA to group BSG samples belonging to the same base malts group with a high percentage of explained variance (75%) was already highlighted by di Matteo et al. [
73].
Figure 3 shows a biplot of Factors 1 and 2 that accounted for 67.63% of the variance in the whole data set. Regarding the projection of the BSG samples on the factorial plan (
Figure 3a), Em samples differed from the others for their negative loading of Factor 1, accounting for 43.19% of the explained variance. Em samples were furtherly divided into two geographically homogeneous groups, one including spent grains from Daunia and another including spent grains from Salento, characterised by the negative and positive loading of Factor 2, respectively. The negative loadings of this factor were associated with high amounts of ash and most of the phenolic compounds while the positive loadings of these factors were associated with high amounts of insoluble fibres, unextractable proteins,
p-coumaric acid, and epigallocatechingallate (
Figure 3b). Ri-D and Ri-S spent grains were homogeneously grouped but close to each other in the upper right quadrant, the first with loadings near to 0, the latter with more positive loadings of Factor 1 (
Figure 3a), characterised by high antioxidant activity values and low phenolic concentrations (
Figure 2b). Da-D and Da-S spent grains were homogeneously grouped but close to each other in the lower right quadrant (
Figure 3a). This quadrant was associated with low-to-intermediate values of all variables (
Figure 3b).
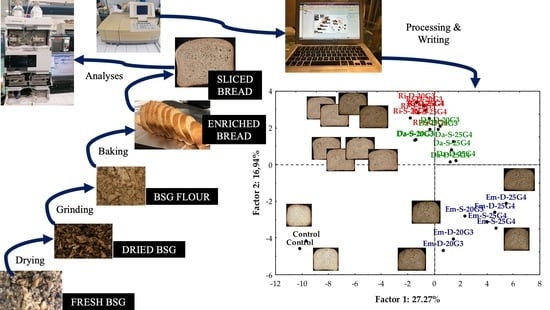
Principal Component Analysis was also performed to highlight the relationship between bread samples and their chemical, physical, structural, and sensory characteristics.
Figure 4a,b shows the projection on the factorial plan of breads and analytical information, respectively. Factors 1 and 2 only explained 44.21% of the variance in the whole data set and made it possible to group the thirteen types of samples into just three sets that stood out for their position in the factorial plan: one including the control bread, placed in the lower left quadrant and associated with high values of crumb development, specific volume, minimum and maximum height, cohesiveness, and amount of
p-coumaric acid; another group, comprising all the Em breads distributed within the lower right quadrant and characterised by high concentrations of IDF, TPC, gallic acid, vanillic acid, sinapic acid, epigallocatechingallate, and high values of crispiness, porosity, fibrousness, and wheat smell; and the last group, including Ri and Da breads, which were partially overlapped and concentrated straddling the two upper quadrants, associated with high values of overall smell intensity, a toasty smell, pore size, crust thickness, overall quality, a high concentration of caffeic acid, a darker crumb colour, and intermediate TFC. As can be inferred from
Figure 4a, PCA of the overall data set was not able to discriminate bread samples on the base of the two different percentages of the BSG-gluten used in bread-making. In the paper of Ktenioudaki et al. [
16], PCA analysis of volatile compounds was able to clear separate snack samples in homogeneous clusters for the percentage of BSG added but it is appropriate to point out that they worked with a single type of BSG instead of three types as we did.
Pearson correlation coefficients were calculated to individuate correlations among the quality characteristics of breads and measured variables. For the sake of synthesis, only the main statistically significant correlations (p < 0.01) are discussed.
The percentage of unextractable proteins was positively correlated with the crumb pore size (+0.61) and negatively correlated with porosity (−0.56), sensory crust hardness (−0.53), and antioxidant activity (−0.60). A possible explanation of this behaviour is that the formation of larger protein aggregates made the crumb structure more compact and entrapped phenolic compounds through protein-polyphenol complexation while increasing the water holding capacity of the crust structure.
The insoluble dietary fibre content was positively correlated with ash content (+0.83), porosity (+0.47), and crumb fibrousness (+0.51) while the soluble dietary fibre content was positively correlated with crust thickness (+0.51) and crumb sweetness (+0.70) as a consequence of the depolymerization of hemicellulose and cellulose and the production of simple sugars. To confirm the significant effects of the addition of BSGs in terms of colour changes, IDF% resulted in a significant correlation with crust b* (−0.54), crumb a* (+0.58), and the colour of the crust (+0.79) and crumb (+0.62). However, contrary to what Ginindza et al. [
68] highlighted, the darkening of the colour did not negatively affect the overall quality of the bread. IDF% as an index of the amount of BSG added also affected bread taste by reducing saltiness (−0.82) and olfactory characteristics, and enhancing the intensity of the wheat (+0.80) and yeast (+0.58) smell. Contrary to what Ginindza et al. [
68] highlighted, the flavour changes caused by the addition of BSG were not correlated with the overall sensory quality of bread.
TPC was positively correlated with ash content (+0.66), IDF% (+0.74), antioxidant activity (+0.70), and with the individual concentration of most of the phenolic compounds, thus demonstrating that wholegrain derivatives can be considered good sources of phenolic antioxidants.
Further significant correlations were found between the physical, structural, and sensory characteristics of breads, as in the following cases: crumb specific volume and crumb development (+0.43); porosity and crumb pore size (−0.48); crust b* and crust colour (−0.51); and crumb L*, a*, and b* and crumb colour (−0.38, +0.68, +0.43).
Finally, the overall quality of breads was positively correlated with a malty smell (0.68) and crumb saltiness (0.70), and negatively correlated with ash and IDF% (−0.72 and −0.52), and with crumb fibrousness and porosity (−0.56 and −0.58). Our results were only partially in agreement with the findings of Ktenioudaki et al. [
16], who found that taste and texture were the attributes that mostly affected the overall acceptability, and with the results of Combest and Warren [
74], who found significant correlations only between taste and overall liking. In fact, in our study, some smell, taste, and texture attributes are correlated with the overall sensory quality of breads.