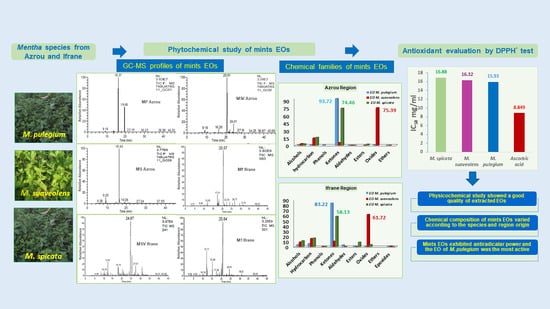
Physicochemical Characterization and Antioxidant Properties of Essential Oils of M. pulegium (L.), M. suaveolens (Ehrh.) and M. spicata (L.) from Moroccan Middle-Atlas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Phytochemical Study
2.2.1. Quality Control of the Dry Matter
Moisture Content
- M0: initial mass of the plant;
- M1: mass after drying.
Determination of the pH
Ash Content
- OM%: organic matter;
- W1: weight of the capsule and sample before calcination;
- W2: weight of the capsule and the sample after calcination;
- TS: test sample.
Determination of the Acidity
- M: mass in grams of the product taken;
- V0: volume in milliliters of the test sample;
- V1: volume in milliliters of the potassium hydroxide solution at the 0.1 N used.
2.2.2. Quality Control of the Mint Species EOs
Determination of the Brix Degree
Determination of the Acid Value (AV)
Determination of the Iodine Value
- B: volume (mL) of 0.1 N sodium thiosulphate required by blank;
- S: volume (mL) of 0.1 N sodium thiosulphate required by sample;
- N: normality of sodium thiosulphate solution.
Determination of the Peroxide Index
- N: normality of sodium thiosulphate solution.
2.2.3. Extraction and GC-MS Analysis of the Mint EOs
- W(EO): weight of the recovered EO (g);
- WO: weight of the plant material (100 g).
2.3. In Vitro Tests of the Antioxidant Activity of the EOs Using a DPPH• Assay
- AA%: percentage of antioxidant activity;
- Acontrol: absorbance of the solution containing only radical DPPH• solution;
- Asample: absorbance of the sample solution to be tested in the presence of DPPH•.
2.4. Data Analysis
3. Results and Discussion
3.1. Phytochemical Study
3.1.1. Physicochemical Properties of the Dry Matter
3.1.2. Physicochemical Properties of the Mentha EOs
3.1.3. The Yield of the Mint EOs
3.1.4. Chemical Compositions of the Mentha Species EOs
Chemical Composition of the M. Pulegium EOs
Chemical Composition of the M. Suaveolens EOs
Chemical Composition of the M. Spicata EOs
3.2. Antioxidant Activity of the Mint EOs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals. Antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar]
- McCall, M.R.; Frei, B. Can antioxidant vitamins materially reduce oxidative damage in humans? Free. Radic. Biol. Med. 1999, 26, 1034–1053. [Google Scholar]
- Sanhueza, J.; Nieto, S.; Valenzuela, A. Thermal stability of some commercial synthetic antioxidants. J. Am. Oil Chem. Soc. 2000, 77, 933–936. [Google Scholar] [CrossRef]
- Amoratti, R.; Mario, C.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar]
- Chang, S.; Abbaspour, H.; Nafchi, A.M.; Heydari, A.; Hosseini, M. Iranian Foeniculum vulgare essential oil and alcoholic extracts: Chemical composition, antimicrobial, antioxidant and application in olive oil preservation. J. Essent. Oil Bear Plants 2016, 19, 1920–1931. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar]
- Sarikurkcu, C.; Eryigit, F.; Cengiz, M. Screening of the Antioxidant Activity of the Essential Oil and Methanol Extract of Mentha pulegium L. From Turkey. Spectrosc. Lett. 2012, 45, 352–358. [Google Scholar] [CrossRef]
- NF V03-402; Epices et Aromates—Détermination de la Teneur en Eau—Méthode par Entrainement. Afnor Editions: Paris, France, 1985.
- NF V 05-108; Produits de L’agriculture. Détermination Conventionnelle du Potentiel Hydrogène. Afnor Editions: Paris, France, 1970.
- NF V 05-113; Détermination des Cendres Totales. Afnor Editions: Paris, France, 1972; Volume 49, pp. 289–298.
- NF V 05-101; Produits Dérivés des Fruits et Légumes. Détermination de l’acidité Titrable. Afnor Editions: Paris, France, 1974.
- Quoc, L.P.T. Physicochemical properties and antibacterial activity of essential oil of Ageratum conyzoides L. leaves. Agric. Conspec. Sci. 2020, 85, 139–144. [Google Scholar]
- Manuranjani, G.; Lalduhsanga, P.; Lalhlenmawia, H.; Bibhuti, K.; Tazami, K. Physicochemical, Antibacterial and Antioxidant Properties of Fixed and Essential Oils Extracted from the Peels of Citrus macroptera Fruit. Indian J. Pharm. Sci. 2019, 81, 82–88. [Google Scholar] [CrossRef]
- Nikhat, F.; Satynarayana, D.; Subhramanyam, E.V.S. Isolation, characterization and screening of antioxidant activity of the roots of Syzygium cuminii (L.) Skeel. Asian J. Res. Chem. 2009, 2, 218–221. [Google Scholar]
- Jensen, D.R.; Thomas, L. Soil pH and the Availability of Plant Nutrients. IPNI Plant Nutr. Today 2010, 2. Available online: http://www.ipni.net/publication/pnt-na.nsf/0/013F96E7280A696985257CD6006FB98F/$FILE/PNT-2010-Fall-02.pdf (accessed on 6 February 2023).
- Tanavar, H.; Barzegar, H.; Behbahani, B.A.; Mehrnia, M.A. Investigation of the chemical properties of Mentha pulegium essential oil and its application in O. basilicum seed mucilage edible coating for extending the quality and shelf life of veal stored in refrigerator (4 °C). Food Sci. Nutr. 2021, 9, 5600–5615. [Google Scholar] [PubMed]
- Taraseviciené, Z.; Velicka, A.; Jariené, E.; Paulauskiené, A.; Kieltyka, D.; Adasiewicza, A.; Sawicka, B.; Gajewski, M. Comparison of Chemical Composition and Colour Parameters of Different Mentha Genus Plants Grownunder Organic Conditions. Not. Bot. Horti. Agrobo. 2019, 47, 92–99. [Google Scholar]
- Sulieman, A.E.; Abdelrahman, S.E.; Rahim, A.M.A. Phytochemical Analysis of Local Spearmint (Mentha spicata) Leaves and Detection of the Antimicrobial Activity of its Oil. J. Microbiol. Res. 2011, 1, 1–4. [Google Scholar] [CrossRef]
- Adepoju, O.T.; Onasanya, L.O.; Udoh, C.H. Comparative studies of nutrient composition of cocoyam (Colocassia esculenta) leaf with some green leafy vegetables. Afric. J. Biotechnol. 2006, 4, 497–501. [Google Scholar]
- Dairo, F.A.S.; Adanlawo, I.G. Nutritional Quality of Crassocephalum crepidiodes and Seneciobiafrae. Pak. J. Nutr. 2007, 6, 35–39. [Google Scholar]
- Oduntan, A.O.; Olaleye, O. Effect of Plant Maturity on the Proximate Composition of Sesamum Radiatum Schum Leaves. J. Food Stud. 2012, 1, 69–76. [Google Scholar]
- Momin, R.K.; Kadam, V.B. Determination of ash values of some medicinal plants of genus sesbania of marathwada region in maharashtra. J. Phytol. 2011, 3, 52–54. [Google Scholar]
- Aldogman, B.; Bilel, H.; Moustafa, S.M.N.; Elmassary, K.F.; Ali, H.M.; Alotaibi, F.Q.; Hamza, M.; Abdelgawad, M.A.; El-Ghorab, A.H. Investigation of Chemical Compositions and Biological Activities of Mentha suaveolens L. from Saudi Arabia. Molecules 2022, 27, 2949. [Google Scholar] [CrossRef]
- Saba, I.; Anwar, F. Effect of Harvesting Regions on Physico-chemical and Biological Attributes of Supercritical Fluid-Extracted Spearmint (Mentha spicata L.) Leaves Essential Oil. J. Essent Oil Bear Plants 2018, 21, 400–419. [Google Scholar]
- Suceveanu, M.; Alexa, I.-C.; Patriciu, O.-I.; Grosu, L.; Fînaru, A. Physicochemical characterization and acceptability of some artisanal mint liqueurs. Sci. Study Res. 2018, 19, 203–210. [Google Scholar]
- Radi, F.; Bouhrim, M.; Mechchate, H.; Al-Zahrani, M.; Qurtam, A.M.A.; Aleissa, A.; Drioiche, A.; Handaq, N.; Zair, T. Phytochemical Analysis, Antimicrobial and Antioxidant Properties of Thymus zygis L. and Thymus willdenowii Boiss. Essential Oils. Plants 2022, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Salim, R.A.; Abu-Goukh, A.B.A.; Khalid, H.E.S.; El-Hassan, G.M. Effect of refinery on spearmint (Mentha spicata var. Viridis l) oil quality. J. Food Process. Technol. 2015, 6, 481–487. [Google Scholar]
- Majdinasab, M.; Niakousari, M.; Shaghaghian, S.; Dehghani, H. Antimicrobial and antioxidant coating based on basil seed gumin corporate with Shirazithyme and summer savory essential oils emulsions for shelf-life extension of refrigerated chicken fillets. Food Hydrocoll. 2010, 108, 106011. [Google Scholar]
- Quoc, L.P.T. Physicochemical properties components, and antibacterial activity Mentha arvensis L. leaves. Uchenye Zapiski Kazanskogo Universiteta. Seriya Estestvennye 2022, 1, 36–45. [Google Scholar]
- Amalich, S.; Zerkani, H.; Cherrat, A.; Soro, N.K.; Bourakhouadar, M.; Fadli, M.; Chevalier, J.; Saad, A.; Mezrioui, N.E.; Hassani, L. Essential oils from Moroccan plants as potential chemo-sensitizers restoring antibiotic activity in resistant Gram negative bacteria. Int. J. Antimicrob. Agent. 2016, 38, 325–330. [Google Scholar]
- Zekri, N.; Sabri, H.; Khannouchi, S.; El Belghiti, M.A.; Zair, T. Phytochemical study and fumigant toxicity of Mentha suaveolens Ehrh essential oil from Morocco against adults of S. oryzae (L.). Austr. J. Basic Appl. Sci. 2013, 7, 599–606. [Google Scholar]
- Zekri, N.; Elazzouzi, H.; Drioche, H.; Satrallah, A.; El Belghiti, M.A.; Zair, T. Effect of Geographic Locations on Chemical Composition of M. Spicata (L.) Essential oils from Moroccan Middle-Atlas. Der Pharm. Lett. 2016, 8, 146–150. [Google Scholar]
- Bruneton, J. Pharmacognosie, Phytochimie et Plantes Médicinales, 4th ed.; Technique et Documentation; Lavoisier: Paris, France, 2009; p. 1268. [Google Scholar]
- Chebli, B.; Achouri, M.; Hassani, L.M.I.; Hmamouchi, M. Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea. J. Ethnopharmacol. 2003, 89, 165–169. [Google Scholar]
- Zekri, N.; Amalich, S.; Boughdad, A.; El Belghiti, M.A.; Zair, T. Phytochemical study and insecticidal activity of Mentha pulegium L. oils from Morocco against Sitophilus Oryzae. Mediter. J. Chem. 2013, 2, 607–619. [Google Scholar] [CrossRef]
- Hmiri, S.; Rahouti, M.; Habib, Z.; Satrani, B.; Ghanmi, M.; El Ajjouri, M. Évaluation du potentiel antifongique des huiles essentielles de Mentha pulegium et d’Eucalyptus Camaldulensis dans la lutte biologique contre les champignons responsables de la détérioration des pommes en conservation. Bull. Société R. Sci. Liège 2011, 80, 824–836. [Google Scholar]
- Sbayou, H.; Boumaza, A.; Hilali, A.; Amghar, S. Antioxidant properties of Artemisia herba-alba Asso, Mentha pulegium L. and Origanum compactum Benth. essential oils. J. Mater. Environ. Sci. 2016, 7, 2908–2912. [Google Scholar]
- Zantar, S.; El Garrouj, D.; Pagán, R.; Chabi, M.; Laglaoui, A.; Bakkali, M.; Zerrouk, M.H. Effect of Harvest Time on Yield, Chemical Composition, Antimicrobial and Antioxidant Activities of Thymus vulgaris and Mentha pulegium Essential Oils. Eur. J. Med. Plants 2015, 8, 70–77. [Google Scholar]
- Chraibi, M.; Farah, A.; Lebrazi, S.; El Amin, O.; Houssaini, M.I.; Fikri-Benbrahim, K. Antimycobacterial natural products from Moroccan medicinal plants: Chemical composition, bacteriostatic and bactericidal profile of Thymus satureioides and Mentha pulegium essential oils. Asian Pacific, J. Trop. Biomed. 2016, 6, 836–840. [Google Scholar] [CrossRef] [Green Version]
- Ait-Ouazzou, A.; Lorán, S.; Arakrak, A.; Laglaoui, A.; Rota, C.; Herrera, A.; Pagán, R. Evaluation of the chemical composition and antimicrobial activity of Mentha pulegium, Juniperus phoenicea, and Cyperus longus essential oils from Morocco. Food Res. Int. 2011, 45, 313–319. [Google Scholar]
- Benayad, N. Les Huiles Essentielles Extraites des Plantes Médicinales Marocaines: Moyen Efficace de Lutte Contre les Ravageurs des Denrées Alimentaires Stockées. Ph.D. Thesis, University of Sciences, Rabat, Morocco, 2008; p. 63. [Google Scholar]
- Lamiri, A.; Lhaloui, S.; Benjilali, B.; Berrada, M. Insecticidal effects of Hessian Fly against Mayetiola destructor (Say). Field Crop Res. 2001, 71, 9–15. [Google Scholar]
- Boughdad, A.; Elkasimi, R.; Kharchafi, M. Activité insecticide des huiles essentielles de Mentha Sur Callosobrochus maculatus (F) (Coleoptera, Bruchidea). In Proceedings of the AFPP—Neuvième Conférence Internationale sur les Ravageurs en Agriculture, Montpellier, France, 26–27 October 2011; Volume 9. [Google Scholar]
- El-Ghorab, A.H. The chemical composition of Mentha pulegium (L.) essential oil from Egypt and its antioxidant activity. J. Ess. Oil Bear. Plant 2006, 9, 183–195. [Google Scholar]
- Agnihotri, V.K.; Agarwal, S.G.; Dhar, P.L.; Thappa, R.K.; Kapahi, B.K.; Saxena, R.K.; Qazi, G.N. Essential oil composition of Mentha pulegium growing wild in the north-western Himalayas India. Flavour Fragr. J. 2005, 20, 607–610. [Google Scholar]
- Lorenzo, D.; Paz, D.; Dellacassa, E.; Davies, P.; Vila, R.; Canigueral, S. Essential Oils of Mentha pulegium and Mentha rotundifolia from Uruguay. Braz. Arch. Biol. Technol. 2002, 45, 519–524. [Google Scholar] [CrossRef]
- Beghidja, N.; Bouslimani, N.; Benayache, F.; Banayache, S.; Chalchat, J.C. Composition of the oils from Mentha pulegium Grown in different areas of the east of Algeria. Khimiya Prirodnykh Soedinenii. 2007, 4, 394–395. [Google Scholar] [CrossRef]
- Kokkini, S.; Handilou, E.; Karousou, R.; Lanaras, T. Variations of pulegone content in pennyroyal (Mentha pulegium L.) plants growing wild in Greece. J. Essent. Oil Res. 2002, 14, 224–227. [Google Scholar]
- Zwaving, J.H.; Smith, D. Composition of the essential oil of Austrian Mentha pulegium. Phytochem 1971, 10, 1951–1953. [Google Scholar]
- Mahboubi, M.; Haghi, G. Antimicrobial activity and chemical composition of Mentha pulegium L. essential oil. J. Ethnopharmacol. 2008, 119, 325–329. [Google Scholar] [PubMed]
- Derwich, E.; Benziane, Z.; Taouil, R.; Senhaji, O.; Touzani, M. Comparative Essential oil Composition of Leaf of M. rotundifolia and M. pulegium a Traditional Herbal Medecine in Morocco. Am. Eurasian J. Sustain. Agric. 2010, 4, 47–54. [Google Scholar]
- Baali, F.; Boumerfeg, S.; Napoli, E.; Boudjelal, A.; Righi, N.; Deghima, A.; Baghiani, A.; Ruberto, G. Chemical Composition and Biological Activities of Essential Oils from Two Wild Algerian Medicinal Plants: Mentha pulegium L. and Lavandula stoechas L. J. Essent. Oil Bear. Plants 2019, 22, 821–837. [Google Scholar]
- Mata, A.T.; Proença, C.; Ferreira, A.R.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Araújo, M.E.M. Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices. Food Chem. 2007, 103, 778–786. [Google Scholar] [CrossRef]
- Stoyanova, A.; Georgiev, E.; Kula, J.; Majda, T. Chemical composition of the essential oil of Mentha pulegium L. from Bulgaria. J. Essent. Oil Res. 2005, 17, 475–476. [Google Scholar] [CrossRef]
- Cook, C.M.; Maloupa, E.; Kokkini, S.; Lanaras, T. Differences between the inflorescence, leaf and stem essential oils of wild Mentha pulegium L plants from Zakynthos Greece. J. Essent. Oil Res. 2000, 12, 598–600. [Google Scholar]
- Sticher, O.; Flück, H. Die zusmmensetzung von genuinen, extrahierten und distillierten ätherischen Ölen einiger Mentha-Arten. Pharm. Acta Helv. 1968, 43, 411–446. [Google Scholar]
- Başer, K.H.C.; Kürkcüoglu, M.; Tarimicilar, G.; Kaynak, G. Essential oils of Mentha species from northern Turkey. J. Essent. Oil Res. 1999, 11, 579–588. [Google Scholar]
- Hajlaoui, H.; Trabelsi, N.; Noumi, E.; Snoussi, M.; Fallah, H.; Ksouri, R.; Bakhrouf, A. Biological activities of the essential oils and methanol extract of tow cultivated mint species (M. longifolia and M. pulegium) used in the Tunisian folkloric medicine. World J. Microbiol. Biotechnol. 2009, 25, 2227–2238. [Google Scholar] [CrossRef]
- Aghel, N.; Yamini, Y.; Hadjiakhoondi, A.; Pourmortazavi, S.M. Supercrtical carbon dioxide extraction of Mentha pulegium L. essential oil. Talanta 2004, 62, 407–411. [Google Scholar] [CrossRef]
- Fujita, Y.; Fujita, S.I. Essential oil of Mentha pulegium and M. grattefossei view from the standpoint of comparative biochemistry. Nippon Kagaku Zasshi 1967, 88, 767–768. [Google Scholar]
- Bekka-Hadji, F.; Bombarda, I.; Djoudi, F.; Bakour, S.; Touati, A. Chemical Composition and Synergistic Potential of M. pulegium L. and Artemisia herba alba Asso. Essential Oils and Antibiotic against Multi-Drug Resistant Bacteria. Molecules 2022, 27, 1095. [Google Scholar] [CrossRef] [PubMed]
- Sutour, S. Etude de la Composition Chimique D’huiles Essentielles et D’extraits de Menthes de Corse et de Kumquats. Ph.D. Thesis, Université de Corse Pascal Paoli, Corte, France, 2010; p. 221. [Google Scholar]
- Ez-Zriouli, R.; El Yacoubi, H.; Imtara, H.; El-Hessni, A.; Mesfioui, A.; Tarayrah, M.; Mothana, R.A.; Noman, O.M.; Mouhsine, F.; Rochdi, A. Chemical composition and antimicrobial activity of essential oils from Mentha pulegium and R. officinalis against multidrug resistant microbes and their acute toxicity study. Open Chem. 2022, 20, 694–702. [Google Scholar] [CrossRef]
- El Arch, M.; Satrani, B.; Farah, A.; Bennani, L.; Boriky, D.; Fechtal, M.; Blaghen, M.; Talbi, M. Composition chimique et activités antimicrobienne et insecticide de l’huile essentielle de Mentha rotundifolia du Maroc. Acta Bot. Gall. 2003, 150, 267–274. [Google Scholar]
- Oumzil, H.; Ghoulami, S.; Rhajaoui, M.; Ilidrissi, A.; Fkih-Tetouani, S.; Faid, M.; Benjouad, A. Antibacterial and antifungal activity of essential oils of Mentha suaveolens. Phytother. Res. 2002, 16, 727–731. [Google Scholar] [CrossRef]
- Galambosi, B.; Aflatuni, A.; Sorvari, K. Effect of cultivation techniques on mint oils in northern Finland. Perfum. Flavor 1998, 23, 27–31. [Google Scholar]
- De la Torre, C.P.; Torres, O.A. Aceite esencial de Mentha rotundifolia. Arch. Bioquim. Quim. Farm. 1977, 20, 85–88. [Google Scholar]
- Lawrence, M.B. Mint: The genus Mentha. In Medicinal and Aromatic Plants—Industrial Profiles; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2007; p. 556. [Google Scholar]
- Velasco-Negueruela, A.; Perez-Alonso, M.J.; Esteban, J.L.; Vallejo, M.C.G.; Zygaldo, J.A.; Guzman, C.A.; Ariza-Espinar, L. Essential oils of Calamintha nepeta (L.) Savi and Mentha aff suaveolens Ehrh. grown in Cordoba, Argentina. J. Essent. Oil Res. 1996, 8, 81–84. [Google Scholar] [CrossRef]
- Raya, M.D.P.; Utrilla, M.P.; Navarro, M.C.; Jiménez, J. CNS activity of M. Rotundifolia and M. longifolia essential oil in Mice and Rats. Phytother. Res. 1990, 4, 232–234. [Google Scholar] [CrossRef]
- Zerkani, H.; Tagnaoute, I.; Zerkani, S.; Radi, F.Z.; Amine, S.; Cherrat, A.; Drioiche, A.; Zine, N.E.; Benhlimaand, N.; Zair, T. Study of the Chemical Composition, Antimicrobial and Antioxidant Activities of the Essential Oil of M. suaveolens Ehrh from Morocco. J. Essent Oil Bear. Plants 2021, 24, 243–253. [Google Scholar]
- Bouyahya, A.; Belmehdi, O.; Abrini, J.; Dakka, N.; Moussaoui, Y.B. Chemical composition of Mentha suaveolens and Pinus halepensis essential oils and their antibacterial and antioxidant activities. Asian Pac. J. Trop. Med. 2019, 12, 117–122. [Google Scholar] [CrossRef]
- El Moussaoui, N.; Sanchez, G.; Khay, E.O.; Idaomar, M.; Mansour, A.I.; Abrini, J.; Aznar, R. Antibacterial and antiviral activities of essential oils of northern Moroccan plants. Br. Biotechnol. J. 2013, 3, 318–331. [Google Scholar]
- Koliopoulos, G.; Pitarokili, D.; Kioulos, E.; Michaelakis, A.; Tzakou, O. Chemical composition and larvicidal evaluation of Mentha, Salvia, and Melissa essential oils against the West Nile virus mosquito Culex pipiens. Parasitol. Res. 2010, 107, 327–335. [Google Scholar] [CrossRef]
- Brada, M.; Bezzina, M.; Marlier, M.; Carlier, A. Variabilité de la composition chimique des huiles essentielles de Mentha rotundifolia du nord de l’Algérie. Biotechnol. Agron. Soc. Environ. 2007, 11, 3–7. [Google Scholar]
- Aziz, E.E.; Abbas, M.H. Chemical Composition and Efficiency of Five Essential Oils against the Pulse Beetle Callosobruchus maculatus (F.) on Vigna radiata Seeds. Am.-Eurasian J. Agric. Environ. Sci. 2010, 8, 411–419. [Google Scholar]
- Gimenez-Santamarina, S.J.A.; Llorens-Molina, F.; Sempere-Ferre, C.; Santamarina, J.; Rosellóand, M.; Santamarina, P. Chemical composition of essential oils of three Mentha species and their antifungal activity against selected phytopathogenic and post-harvest fungi. All Life 2022, 15, 64–73. [Google Scholar] [CrossRef]
- Pino, J.A.; Rosada, A.; Fuentes, V. Chemical composition of the leaf of Mentha rotundifolia (L.) Hudson from Cuba. J. Essent. Oil. Res. 1999, 11, 241–242. [Google Scholar]
- Avato, P.; Sgarra, G.; Casadoro, G. Chemical composition of the essential oils of Mentha species cultivated in Italy. Sci. Pharm. 1995, 63, 223–230. [Google Scholar]
- El Fadl, A.; Chtaina, N. Etude de Base sur la Culture de la Menthe du Maroc. Programme Régional de Lutte Intégrée Contre les Organismes nui.sibles (Integrated Pest Management) au Proche Orient; Office National de Sécurité Sanitaire des Produits Alimentaires (ONSSA): Rabat, Morocco, 2010; Volume 10. [Google Scholar]
- Kee, L.A.; Shori, A.B.; Baba, A.S. Bioactivity and health effects of Mentha spicata. Integr. Food Nutr. Metab. 2017, 5, 1–2. [Google Scholar]
- Misra, L.N.; Tyagi, B.R.; Thakur, R.S. Chemotypic variation in Indian spearmint. Planta. Med. 1989, 55, 575–576. [Google Scholar] [PubMed]
- El Hassani, F.Z.; Zinedine, A.; Alaoui, S.M.; Merzouki, M.; Benmlih, M. Use of olive mill wastewater as an organic amendment for Mentha spicata (L.). Ind. Crops Prod. 2010, 32, 343–348. [Google Scholar] [CrossRef]
- El- Wahab, M.A.A. Evaluation of Spearmint (Mentha spicata L.) Productivity Grown in Different Locations under Upper Egypt Conditions. Res. J. Agric. Biol. Sci. 2009, 5, 250–254. [Google Scholar]
- El Anbri, C.; Eddaya, T.; Boughdad, A.; Chaimbault, P.; Zaid, A. Essential oil chemical diversity of Moroccan mint (Mentha spicata L.). Mor. J. Agric. Sci. 2022, 3, 189–202. [Google Scholar]
- Kelen, M.; Tepe, B. Chemical composition, antioxidant and antimicrobial properties of the essential oils of three Salvia species from Turkish flora. Bioresour. Technol. 2008, 99, 4096–4100. [Google Scholar] [CrossRef]
- Hariri, A.; Ouis, N.; Bouhadi, D.; Benatouche, Z. In vitro antioxidant activity of essential oil of aerial parts of Mentha pulegium L. Acta Agric. Serbica 2020, 25, 193–197. [Google Scholar]
- Proestos, C.; Lytoudi, K.; Mavromelanidou, O.K.; Zoumpoulakis, P.; Sinanoglou, V.J. Antioxidant Capacity of Selected Plant Extracts and Their Essential Oils. Antioxidants 2013, 2, 11–22. [Google Scholar]
- Kamkar, A.; Javan, A.J.; Asadi, F.; Kamalinejad, M. The antioxidative effect of Iranian Mentha pulegium extracts and essential oil in sunflower oil. Food Chem. Toxicol. 2010, 48, 1796–1800. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Batista, I.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. European pennyroyal (Mentha pulegium) from Portugal: Chemical composition of essential oil and antioxidant and antimicrobial properties of extracts and essential oil. Ind. Crop. Prod. 2012, 36, 81–87. [Google Scholar] [CrossRef]
- El-Kashoury, E.A.; El-Askary, H.I.; Kandi, Z.A. Chemical and biological study of Mentha suaveolens (Ehrh.) cultivated in Egypt. J. Med. Plants Res. 2014, 8, 747–755. [Google Scholar]
- El Ansary, H.O.; Ashmawy, N.A. Essential oils of mint between benefits and hazards. J. Essent. Oil Bear. Plants 2013, 16, 429–438. [Google Scholar] [CrossRef]
- Snoussi, M.; Hajlaoui, H.; Noumi, E.; Usai, D.; Sechi, L.A.; Zanetti, S.; Bakhrouf, A. In vitro anti-vibrio spp. activity and chemical composition of some Tunisian plants. World J. Microbiol. Biotechnol. 2008, 24, 3071–3076. [Google Scholar]
- Dhifi, W.; Jelali, N.; Mnif, W.; Litaiem, M.; Hamdi, N. Chemical composition of the essential oil of Mentha spicata from Tunisia and its biological activities. J. Food Biochem. 2013, 37, 362–368. [Google Scholar]
- Mkaddem, M.; Bouajila, J.; Ennajar, M.; Lebrihi, A.; Mathieu, F.; Romdhane, M. Chemical Composition and Antimicrobial and Antioxidant Activities of Mentha (longifolia L. and viridis) Essential Oils. J. Food Sci. 2009, 74, 358–363. [Google Scholar]
- Martins, M.R.; Tinoco, M.T.; Almeida, A.S.; Cruz-Morais, J. Chemical composition, antioxidant and antimicrobial properties of three essential oils from Portuguese flora. J. Pharmacogn. 2012, 3, 39–44. [Google Scholar]
- Laghouiter, O.K.; Ghzrib, A.; Laghouiter, H. Etude de l’activité antioxydante des huiles essentielles de certaines menthes cultivées dans la région de Ghardaïa. El Wahat Pour Rech. Les Etudes 2015, 8, 84–93. [Google Scholar]
- Alsaraf, S.; Hadi, Z.; Jawaid, M.D.; Shah, A.; Khan, A. Chemical profiling, cytotoxic and antioxidant activity of volatile oil isolated from the mint (Mentha spicata L.;) grown in Oman. Biocatalysis. Agricult. Biotechnol 2021, 34, 102034. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Wink, M.; Setzer, W.N. Radical scavenging and antioxidant activities of essential oil components- an experimental and computational investigation. Nat. Prod. Commun. 2015, 10, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Mimica-Dukic, N.; Bozin, B.; Sokovic, M. Antimicrobial and antioxidant activities of three Mentha species essential oils. Planta Med. 2003, 69, 413–419. [Google Scholar]
- Torres-Martínez, R.; García-Rodríguez, Y.M.; Ríos-Chávez, P. Antioxidant Activity of the Essential Oil and its Major Terpenes of Satureja macrostema (Moc. and Sessé ex Benth.) Briq. Pharmacogn. Mag 2017, 13 (Suppl. S4), 875–880. [Google Scholar]





| Species | pH | MC (%) | Acidity (%) | Ash Content (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Azrou | Ifrane | Azrou | Ifrane | Azrou | Ifrane | Azrou | Ifrane | |
| M. pulegium | 5.45 ± 0.029 | 5.78 ± 0.027 | 16 ± 0.017 | 13.94 ± 0.020 | 0.210 ± 0.002 | 0.294 ± 0.003 | 11.25 ± 0.011 | 11.86 ± 0.013 |
| M. suaveolens | 5.67 ± 0.011 | 5.95 ± 0.016 | 17 ± 0.013 | 23.44 ± 0.016 | 0.590 ± 0.003 | 0.608 ± 0.004 | 11.43 ± 0.013 | 11.72 ± 0.022 |
| M. spicata | 5.58 ± 0.013 | 5.82 ± 0.022 | 20 ± 0.009 | 46.67 ± 0.013 | 0.163 ± 0.001 | 0.185 ± 0.002 | 18.89 ± 0.016 | 21.87 ± 0.018 |
| Parameters | M. pulegium EO | M. suaveolens EO | M. spicata EO | |||
|---|---|---|---|---|---|---|
| Azrou | Ifrane | Azrou | Ifrane | Azrou | Ifrane | |
| Brix index (°B) | 72.8 ± 0.099 | 79.23 ± 0.111 | 80.7 ± 0.212 | 81.43 ± 0.222 | 71.6 ± 0.130 | 72.30 ± 0.133 |
| Acidity index (mg KOH/g) | 21.91 ± 0.008 | 28.07 ± 0.011 | 22.03 ± 0.019 | 28.06 ± 0.029 | 26.1 ± 0.036 | 27.10 ± 0.038 |
| Iodine index (g I/100 g) | 110.160 ± 0.001 | 110.17 ± 0.001 | 107.354 ± 0.018 | 106.61 ± 0.019 | 103.759 ± 0.001 | 111.12 ± 0.002 |
| Peroxide value (m Eq O2/kg) | 12.01 ± 0.136 | 14.03 ± 0.178 | 20.9 ± 0.129 | 21.60 ± 0.133 | 18.4 ± 0.172 | 19.27 ± 0.178 |
| Compound | Calculated RI | MP Area (%) | |
|---|---|---|---|
| Azrou | Ifrane | ||
| α-pinene | 939 | 0.17 | 0.35 |
| β-pinene | 979 | 0.15 | - |
| Myrcene | 987 | - | 0.48 |
| Meta-mentha-1(7), 8-diene | 1000 | 0.02 | - |
| O-cymene | 1026 | 0.07 | - |
| Octanol<3-> | 1027 | - | 0.77 |
| Limonene | 1029 | 0.90 | 1.64 |
| Cineole<1,8-> | 1031 | - | 0.23 |
| Para-mentha-3,8-diene | 1072 | 0.01 | 0.17 |
| Thujone<Trans-> | 1136 | - | 0.13 |
| Menth-2-en-1-ol<Trans-ρ-> | 1140 | 0.57 | - |
| Octanolacetate<3-> | 1143 | - | 0.08 |
| Benzylacetate | 1162 | 0.07 | - |
| Chrysanthenol<cis-> | 1164 | 1.03 | - |
| Menthone<iso-> | 1170 | - | 1.66 |
| Menth-2-en-1-ol <cis-ρ-> | 1184 | - | 1.09 |
| Terpineol< α> | 1188 | 0.17 | - |
| Menthone | 1198 | - | 5.03 |
| Levomenthol | 1203 | - | 1.37 |
| Cyclocitral<β-> | 1212 | - | 3.49 |
| Pulegol<Trans> | 1214 | 0.19 | - |
| Coahuilensolmethylether | 1221 | 0.16 | - |
| Verbenone | 1249 | - | 0.1 |
| Pulegone | 1237 | 68.86 | 70.92 |
| Piperitone | 1252 | 0.07 | - |
| Piperitoneepoxide<cis-> | 1255 | - | 0.1 |
| Perilla-aldehyde | 1271 | 0.21 | - |
| Thymol | 1290 | 1.01 | - |
| Carvacrol | 1299 | 0.04 | -- |
| Menthylacetate | 1300 | - | 1.55 |
| Piperitoneepoxide<trans-> - | 1308 | - | 0.42 |
| p-vinyl-guaiacol | 1309 | 0.13 | - |
| Menth-1-en-9-ol <ρ-> | 1316 | - | 0.08 |
| Tetrahydrojasmone<trans-> | 1339 | - | 1.89 |
| Piperitenone | 1343 | 24.81 | 2.03 |
| Piperitoneepoxide<trans-> | 1355 | - | 0.26 |
| Caryophyllene<Z-> | 1408 | 0.11 | - |
| Caryophyllene<E> | 1419 | 0.04 | 1.19 |
| α–Guaiene | 1439 | 0.08 | - |
| Ionol<α-(E)-> | 1380 | - | 0.59 |
| Ionone <dihydro-α-> | 1435 | - | 0.10 |
| Humulene<α-> | 1454 | - | 2.17 |
| Ionone<Trans-β> | 1486 | - | 0.13 |
| Dihydroagarofurane<4-Epi-Cis > | 1499 | 0.04 | - |
| Germacrene D-4-ol | 1575 | 0.09 | - |
| Nepetalactone<4aα,7α,7aβ-> | 1566 | - | 1.21 |
| Caryophyllene oxyde | 1583 | 0.09 | 0.47 |
| Humulene epoxide II | 1606 | - | 0.28 |
| Himachalol | 1653 | 0.01 | - |
| Oxygenated monoterpenes | 96.89 | 85.39 | |
| Hydrocarbon monoterpenes | 1.34 | 3.41 | |
| Oxygenated sesquiterpenes | 0.19 | 2.8 | |
| Hydrocarbon sesquiterpenes | 0.31 | 3.36 | |
| Others | 0.37 | 5.12 | |
| Total (%) | 99.10 | 100 | |
| Compound | Calculated RI | Area (%) | |
|---|---|---|---|
| Azrou | Ifrane | ||
| α-pinene | 939 | 0.36 | 0.88 |
| Camphene | 954 | - | 0.48 |
| Sabinene | 983 | - | 0.42 |
| β-pinene | 979 | 0.65 | 1.09 |
| Meta-mentha-1(7),8-diene | 1000 | 0.18 | - |
| Myrcene | 1001 | - | 0.44 |
| Octanol<3-> | 1008 | - | 0.3 |
| α-Terpinene | 1017 | 0.07 | 0.71 |
| p-cimene | 1024 | 0.13 | |
| Limonene | 1029 | 1.85 | 3.49 |
| Cineole<1,8-> | 1052 | - | 0.55 |
| γ -terpinene | 1059 | 0.13 | 1.18 |
| Cis-sabinene hydrate | 1070 | 0.53 | 0.67 |
| Trans-sabinene hydrate | 1098 | 0.06 | - |
| 1-octen-3-yl-acetate | 1112 | 0.13 | 0.32 |
| Dehydro-sabinacetone | 1120 | 0.05 | - |
| 4-acetyl-1-methyl cyclohexene | 1137 | 0.08 | - |
| Nopinone | 1140 | 0.05 | - |
| Sabina Ketone | 1158 | - | 0.31 |
| Borneol | 1169 | 0.27 | 2.39 |
| Terpinen-4-ol | 1177 | 0.71 | 2.22 |
| p-cymen-8-ol | 1182 | 0.12 | |
| α-Terpineol | 1188 | 0.25 | 0.77 |
| MethyletherCoahuilensol | 1221 | 0.14 | |
| Pulegone | 1237 | 2.34 | 1.7 |
| Piperitoneepoxide<trans-> | 1221 | - | 1.34 |
| 2-Allyl-p-cresol | 1255 | - | 0.76 |
| CarvoneOxide Cis | 1263 | 0.44 | 0.87 |
| Carvone | 1242 | - | 0.61 |
| Geranial | 1267 | - | - |
| Perillaaldehyde | 1271 | 0.17 | - |
| Carvacrol | 1298 | - | 0.63 |
| Cinnamaldehyde<α-methyl> | 1317 | - | 0.33 |
| Piperitenone | 1343 | 1.17 | 1.19 |
| PiperitenoneOxide | 1368 | 74.69 | 60.3 |
| Daucene | 1381 | 0.11 | - |
| Bourbonene<β-> | 1387 | - | 0.25 |
| β-Elemene | 1390 | 0.16 | - |
| <4a-α,7-β,7a-α>nepetalactone | 1391 | 1.81 | 6.9 |
| Calamenene<cis-> | 1400 | - | 0.68 |
| Longifolene | 1407 | 0.27 | - |
| β-Caryophyllene | 1419 | 1.68 | - |
| Cis-muurola-3,5-diene | 1450 | 0.09 | - |
| Spirolepechinene | 1451 | 0.16 | - |
| Khusimene | 1455 | 0.68 | - |
| cis-cadina-1(6),4-diene | 1463 | 0.81 | - |
| Muurola-4(14),5-diene <cis-> | 1464 | - | 0.7 |
| Germacrene D | 1478 | - | 2.74 |
| γ -Muurolene | 1479 | 5.53 | - |
| γ -Amorphene | 1495 | 0.30 | - |
| Aciphyllene | 1501 | 0.10 | - |
| γ -cadinene | 1513 | 0.11 | - |
| Trans-calamenene | 1522 | 0.77 | - |
| α-cadinene | 1538 | 0.09 | - |
| Spathulenol | 1578 | 0.60 | 0.31 |
| Oxyde de Caryophellene | 1582 | 0.26 | - |
| Globulol | 1590 | 0.23 | |
| Ledol | 1602 | - | - |
| Viridiflorol | 1603 | - | 0.86 |
| Cubenol<1-epi-> | 1618 | - | 0.46 |
| 1,10-di-epi-Cubenol | 1619 | 0.43 | - |
| Chamazulene | 1625 | - | 2.78 |
| 10-epi-α-cadinol | 1640 | 0.28 | - |
| Torreyol | 1646 | 0.05 | - |
| α-cadinol | 1654 | 0.35 | 0.37 |
| Germacra-4 (15), 5,10(14) trien-1-α-ol | 1686 | 0.07 | - |
| Shyobunol | 1689 | 0.10 | - |
| Oxygenated monoterpenes | 82.83 | 82.69 | |
| Hydrocarbon monoterpenes | 3.37 | 7.51 | |
| Oxygenated sesquiterpenes | 2.37 | 2 | |
| Hydrocarbon sesquiterpenes | 10.86 | 7.15 | |
| Others | 0.18 | 0.65 | |
| Total (%) | 99.61 | 100 | |
| Compound | Calculated RI | Area (%) | |
|---|---|---|---|
| Azrou | Ifrane | ||
| α-pinene | 933 | 0.37 | 0.56 |
| Sabinene | 983 | - | 0.27 |
| β-pinene | 975 | 0.58 | 0.65 |
| Myrcene | 1001 | - | 0.26 |
| Octanol<3-> | 1027 | - | 0.24 |
| Cineole 1,8 | 1028 | - | 2.45 |
| Limonene | 1029 | 10.50 | 9.69 |
| p-mentha-3,8-diene | 1065 | 0.79 | 2.45 |
| Sabinene hydrate <cis-> | 1070 | - | 0.36 |
| Linalooloxide<cis-> | 1095 | - | 0.15 |
| Terpinolene | 1097 | 0.10 | - |
| Linalool | 1098 | - | 5.59 |
| Menthone | 1152 | - | 1.49 |
| Menthone<iso-> | 1153 | - | 0.19 |
| Borneol | 1165 | 0.78 | 0.70 |
| Terpinen-4-ol | 1177 | 0.65 | 0.56 |
| α-terpineol | 1191 | 0.12 | - |
| Trans-4-caranone | 1195 | 2.74 | - |
| Trans-carveol | 1227 | 0.22 | 3.54 |
| Dihydrocarvone cis | 1202 | - | 1.66 |
| Dihydrocarveolneo | 1203 | - | 0.43 |
| Pulegone | 1238 | 0.16 | - |
| Carvone | 1242 | 71.56 | 54.79 |
| Linaloolacetate | 1251 | - | 7.47 |
| p-cymen-7-ol | 1287 | 0.08 | - |
| Carvoneoxide | 1262 | - | 0.42 |
| Thymol | 1290 | - | 0.14 |
| ɣ-Terpinen-7-al | 1292 | 0.10 | - |
| Carvacrol | 1299 | - | 0.41 |
| Iso-dihydrocarveolacetate | 1329 | 2.07 | 0.57 |
| Trans-carvylacetate | 1342 | - | 0.25 |
| cis-carvylacetate | 1364 | 0.44 | 0.52 |
| α-Yalengene | 1375 | 0.08 | - |
| β-Bourbonene | 1384 | 1.04 | 2.84 |
| β-Elemene | 1391 | 0.09 | 0.30 |
| Caryophyllene (E) | 1418 | - | 0.87 |
| β-Caryophyllene | 1418 | 0.76 | - |
| β-Coparene | 1428 | 0.16 | - |
| 6,9-Guaiadiene | 1444 | 0.15 | - |
| Spirolepechinene | 1452 | 0.12 | - |
| Cis-Cadina-1(6),4-diene | 1462 | 0.15 | - |
| Cis-Muurola-4(14),5-diene | 1466 | 0.11 | - |
| Germacrene D | 1479 | 0.61 | 0.35 |
| Muurolene<γ-> | 1468 | - | 0.45 |
| Farnesene<(Z)-β-> | 1476 | - | 0.13 |
| Trans-calamenene | 1522 | 0.33 | 0.35 |
| Spathulenol | 1575 | 0.14 | 0.18 |
| Trans-Sesquisabinene hydrate | 1579 | 0.13 | - |
| Caryophylleneoxide | 1583 | - | 1.04 |
| Humulene epoxide II | 1588 | - | 0.21 |
| Globulol | 1590 | 0.23 | - |
| 1,10-di-epi-Cubenol | 1614 | 0.17 | - |
| Hinesol | 1640 | 0.30 | - |
| α-Cadinol | 1654 | 0.24 | - |
| Oxygenated monoterpenes | 76.41 | 72.88 | |
| Hydrocarbon monoterpenes | 12.34 | 11.67 | |
| Oxygenated sesquiterpenes | 1.21 | 1.43 | |
| Hydrocarbon sesquiterpenes | 3.6 | 5.29 | |
| Others | 2.51 | 8.81 | |
| Total (%) | 96.07 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zekri, N.; Elazzouzi, H.; Ailli, A.; Gouruch, A.A.; Radi, F.Z.; El Belghiti, M.A.; Zair, T.; Nieto, G.; Centeno, J.A.; Lorenzo, J.M. Physicochemical Characterization and Antioxidant Properties of Essential Oils of M. pulegium (L.), M. suaveolens (Ehrh.) and M. spicata (L.) from Moroccan Middle-Atlas. Foods 2023, 12, 760. https://doi.org/10.3390/foods12040760
Zekri N, Elazzouzi H, Ailli A, Gouruch AA, Radi FZ, El Belghiti MA, Zair T, Nieto G, Centeno JA, Lorenzo JM. Physicochemical Characterization and Antioxidant Properties of Essential Oils of M. pulegium (L.), M. suaveolens (Ehrh.) and M. spicata (L.) from Moroccan Middle-Atlas. Foods. 2023; 12(4):760. https://doi.org/10.3390/foods12040760
Chicago/Turabian StyleZekri, Nadia, Hanane Elazzouzi, Atika Ailli, Aman Allah Gouruch, Fatima Zahrae Radi, Mohammed Alaoui El Belghiti, Touriya Zair, Gema Nieto, Juan A. Centeno, and José M. Lorenzo. 2023. "Physicochemical Characterization and Antioxidant Properties of Essential Oils of M. pulegium (L.), M. suaveolens (Ehrh.) and M. spicata (L.) from Moroccan Middle-Atlas" Foods 12, no. 4: 760. https://doi.org/10.3390/foods12040760