Lipidomic and Proteomic Profiling of the Milk Fat Globule Membrane from Different Industrial By-Products of the Butter and Butter Oil Manufacturing Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagent
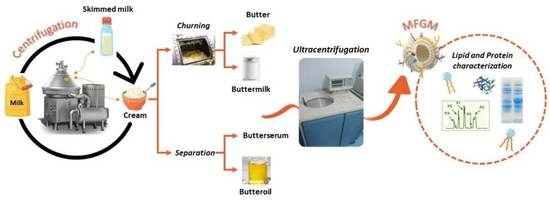
2.2. Isolation of MFGM Fraction
2.3. Analysis of Lipid Fraction
2.3.1. Lipid Extraction
2.3.2. Analysis of Lipid Classes by HPLC-ELSD
2.3.3. Triacylglycerides and Cholesterol Determination Using GC-FID
2.3.4. Determination of Fatty Acid Methyl Esters (FAMEs) Using GC-FID
2.4. Protein Characterization
2.4.1. Protein Content Quantification via Bradford Method
2.4.2. Protein Identification via SDS-PAGE
2.4.3. Protein Identification via MALDI-TOF/TOF
2.5. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Lipid Classes and Protein Characterization of Raw Materials and By-Products of Butter and Butter Oil Processing
3.2. Analysis of Fatty acid Composition
3.3. Triacylglycerols Determination
3.4. Protein Identification via SDS-PAGE
3.5. Lipid and Protein Characterization of MFGM Isolates
3.5.1. Fat and Protein Content of MFGM Isolates
3.5.2. Analysis of Lipid Classes of the Selected Samples and Their Corresponding MFGM Isolates
3.5.3. Fatty Acid Methyl Esters Analysis of the Selected Samples and Their Corresponding MFGM Isolates
3.5.4. Triacylglyceride Determination of the Selected Samples and Their Corresponding MFGM Isolates
3.5.5. Protein Characterization of the Selected Samples and Their Corresponding MFGM Isolates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, F.; Wang, J.; Yu, J. Effect of Cumin Essential Oil (Cuminum Cyminum L.) on Milk Fat Globule Membrane Stability and Micro Structure Properties after Heat Treatment. Int. J. Dairy Sci. 2018, 13, 22–29. [Google Scholar] [CrossRef]
- Holzmüller, W.; Kulozik, U. Quantification of MFGM Proteins in Buttermilk and Butter Serum by Means of a Stain Free SDS-PAGE Method. J. Food Compos. Anal. 2016, 49, 102–109. [Google Scholar] [CrossRef]
- Ortega-Anaya, J.; Jiménez-Flores, R. Symposium Review: The Relevance of Bovine Milk Phospholipids in Human Nutrition—Evidence of the Effect on Infant Gut and Brain Development. J. Dairy Sci. 2019, 102, 2738–2748. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Padhi, E.; Hasegawa, Y.; Larke, J.; Parenti, M.; Wang, A.; Hernell, O.; Lönnerdal, B.; Slupsky, C. Compositional Dynamics of the Milk Fat Globule and Its Role in Infant Development. Front. Pediatr. 2018, 6, 313. [Google Scholar] [CrossRef] [PubMed]
- Manoni, M.; Di Lorenzo, C.; Ottoboni, M.; Tretola, M.; Pinotti, L. Comparative Proteomics of Milk Fat Globule Membrane (MFGM) Proteome across Species and Lactation Stages and the Potentials of MFGM Fractions in Infant Formula Preparation. Foods 2020, 9, 1251. [Google Scholar] [CrossRef]
- Almeida, C.C.; Mendonça Pereira, B.F.; Leandro, K.C.; Costa, M.P.; Spisso, B.F.; Conte-Junior, C.A. Bioactive Compounds in Infant Formula and Their Effects on Infant Nutrition and Health: A Systematic Literature Review. Int. J. Food Sci. 2021, 8850080. [Google Scholar] [CrossRef]
- Raza, G.S.; Herzig, K.-H.; Leppäluoto, J. Invited Review: Milk Fat Globule Membrane—A Possible Panacea for Neurodevelopment, Infections, Cardiometabolic Diseases, and Frailty. J. Dairy Sci. 2021, 104, 7345–7363. [Google Scholar] [CrossRef]
- Fontecha, J.; Brink, L.; Wu, S.; Pouliot, Y.; Visioli, F.; Jiménez-Flores, R. Sources, Production, and Clinical Treatments of Milk Fat Globule Membrane for Infant Nutrition and Well-Being. Nutrients 2020, 12, 1607. [Google Scholar] [CrossRef]
- Le, T.T.; van Camp, J.; Pascual, P.A.L.; Meesen, G.; Thienpont, N.; Messens, K.; Dewettinck, K. Physical Properties and Microstructure of Yoghurt Enriched with Milk Fat Globule Membrane Material. Int. Dairy J. 2011, 21, 798–805. [Google Scholar] [CrossRef]
- Phan, T.T.Q.; Moens, K.; Le, T.T.; Van der Meeren, P.; Dewettinck, K. Potential of Milk Fat Globule Membrane Enriched Materials to Improve the Whipping Properties of Recombined Cream. Int. Dairy J. 2014, 39, 16–23. [Google Scholar] [CrossRef]
- Phan, T.T.Q.; Le, T.T.; Van de Walle, D.; Van der Meeren, P.; Dewettinck, K. Combined Effects of Milk Fat Globule Membrane Polar Lipids and Protein Concentrate on the Stability of Oil-in-Water Emulsions. Int. Dairy J. 2016, 52, 42–49. [Google Scholar] [CrossRef]
- Ali, F.; Tian, K.; Liu, X.; Atehli, D.; Wang, J. Sodium Hexametaphosphate’s Impact on Hydrodynamic Parameters of Milk Fat Globule Membrane. Int. J. Dairy Technol. 2021, 74, 63–74. [Google Scholar] [CrossRef]
- Lopez, C. Valorization of Dairy By-Products for Functional and Nutritional Applications: Recent Trends toward the Milk Fat Globule Membrane. In Valorization Agri-Food Wastes by-Products Recent Trends, Innovations and Sustainability Challenges; Academic Press: Cambridge, MA, USA, 2021; pp. 415–423. [Google Scholar] [CrossRef]
- Oliveira, D.; O’Mahony, J.A. Composition, Fractionation, Techno-Functional Properties and Applications of Milk Fat Globule Membrane–Derived Material. In Advanced Dairy Chemistry, Volume 2; Springer International Publishing: Cham, Switzerland, 2020; Volume 2, pp. 169–195. [Google Scholar] [CrossRef]
- McPherson, A.; Kitchen, B. The Proteins and Lipids of the Aqueous Phase of Butter. Aust. J. Dairy Technol. 1981, 36, 17–20. [Google Scholar]
- Calvo, M.V.; Martín-Hernández, M.C.; García-Serrano, A.; Castro-Gómez, M.P.; Alonso-Miravalles, L.; García-Martín, R.; Megino-Tello, J.; Alonso, L.; Fontecha, J. Comprehensive Characterization of Neutral and Polar Lipids of Buttermilk from Different Sources and Its Milk Fat Globule Membrane Isolates. J. Food Compos. Anal. 2020, 86, 103386. [Google Scholar] [CrossRef]
- Venkat, M.; Chia, L.W.; Lambers, T.T. Milk Polar Lipids Composition and Functionality: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2022, 1–45. [Google Scholar] [CrossRef]
- Da Silva, R.C.; Colleran, H.L.; Ibrahim, S.A. Milk Fat Globule Membrane in Infant Nutrition: A Dairy Industry Perspective. J. Dairy Res. 2021, 88, 105–116. [Google Scholar] [CrossRef]
- Vanderghem, C.; Bodson, P.; Danthine, S.; Paquot, M.; Deroanne, C.; Blecker, C. Milk Fat Globule Membrane and Buttermilks: From Composition to Valorization. Biotechnol. Agron. Soc. Environ. 2010, 14, 485–500. [Google Scholar]
- Bourlieu, C.; Cheillan, D.; Blot, M.; Daira, P.; Trauchessec, M.; Ruet, S.; Gassi, J.Y.; Beaucher, E.; Robert, B.; Leconte, N.; et al. Polar Lipid Composition of Bioactive Dairy Co-Products Buttermilk and Butterserum: Emphasis on Sphingolipid and Ceramide Isoforms. Food Chem. 2018, 240, 67–74. [Google Scholar] [CrossRef]
- Dewettinck, K.; Rombaut, R.; Thienpont, N.; Le, T.T.; Messens, K.; Van Camp, J. Nutritional and Technological Aspects of Milk Fat Globule Membrane Material. Int. Dairy J. 2008, 18, 436–457. [Google Scholar] [CrossRef]
- Roesch, R.R.; Rincon, A.; Corredig, M. Emulsifying Properties of Fractions Prepared from Commercial Buttermilk by Microfiltration. J. Dairy Sci. 2004, 87, 4080–4087. [Google Scholar] [CrossRef]
- Ye, A.; Singh, H.; Taylor, M.W.; Anema, S. Interactions of Whey Proteins with Milk Fat Globule Membrane Proteins during Heat Treatment of Whole Milk. Lait 2004, 84, 269–283. [Google Scholar] [CrossRef]
- Corredig, M.; Roesch, R.R.; Dalgleish, D.G. Production of a Novel Ingredient from Buttermilk. J. Dairy Sci. 2003, 86, 2744–2750. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Blot, M.; Briard-Bion, V.; Cirié, C.; Graulet, B. Butter Serums and Buttermilks as Sources of Bioactive Lipids from the Milk Fat Globule Membrane: Differences in Their Lipid Composition and Potentialities of Cow Diet to Increase n-3 PUFA. Food Res. Int. 2017, 100, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Debyser, G.; Gilbert, W.; Struijs, K.; Van Camp, J.; Van de Wiele, T.; Devreese, B.; Dewettinck, K. Distribution and Isolation of Milk Fat Globule Membrane Proteins during Dairy Processing as Revealed by Proteomic Analysis. Int. Dairy J. 2013, 32, 110–120. [Google Scholar] [CrossRef]
- Qu, X.; Hu, H.; Wang, Y.; Cao, C.; Li, H.; Liu, X.; Yu, J. Proteomics Analysis of Milk Fat Globule Membrane Enriched Materials Derived from By-Products during Different Stages of Milk-Fat Processing. LWT 2019, 116, 108531. [Google Scholar] [CrossRef]
- Corredig, M.; Dalgleish, D.G. Isolates from Industrial Buttermilk: Emulsifying Properties of Materials Derived from the Milk Fat Globule Membrane. J. Agric. Food Chem. 1997, 45, 4595–4600. [Google Scholar] [CrossRef]
- Castro-Gómez, M.P.; Rodriguez-Alcalá, L.M.; Calvo, M.V.; Romero, J.; Mendiola, J.A.; Ibañez, E.; Fontecha, J. Total Milk Fat Extraction and Quantification of Polar and Neutral Lipids of Cow, Goat, and Ewe Milk by Using a Pressurized Liquid System and Chromatographic Techniques. J. Dairy Sci. 2014, 97, 6719–6728. [Google Scholar] [CrossRef]
- Fontecha, J.; Goudjil, H.; Ríos, J.J.; Fraga, M.J.; Juárez, M. Identity of the Major Triacylglycerols in Ovine Milk Fat. Int. Dairy J. 2005, 15, 1217–1224. [Google Scholar] [CrossRef]
- Golay, P.A.; Dionisi, F.; Hug, B.; Giuffrida, F.; Destaillats, F. Direct Quantification of Fatty Acids in Dairy Powders with Special Emphasis on Trans Fatty Acid Content. Food Chem. 2007, 101, 1115–1120. [Google Scholar] [CrossRef]
- Costa, M.R.; Elias-Argote, X.E.; Jiménez-Flores, R.; Gigante, M.L. Use of Ultrafiltration and Supercritical Fluid Extraction to Obtain a Whey Buttermilk Powder Enriched in Milk Fat Globule Membrane Phospholipids. Int. Dairy J. 2010, 20, 598–602. [Google Scholar] [CrossRef]
- Rombaut, R.; Van Camp, J.; Dewettinck, K. Phospho- and Sphingolipid Distribution during Processing of Milk, Butter and Whey. Int. J. Food Sci. Technol. 2006, 41, 435–443. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Codex Standard for Creams and Prepared Creams, Codex STAN. A-9-1976, Rev. 1-2003; FAO/WHO: Rome, Italy, 2003.
- Pugliese, A.; Cabassi, G.; Chiavaro, E.; Paciulli, M.; Carini, E.; Mucchetti, G. Physical Characterization of Whole and Skim Dried Milk Powders. J. Food Sci. Technol. 2017, 54, 3433–3442. [Google Scholar] [CrossRef] [PubMed]
- Gassi, J.Y.; Blot, M.; Beaucher, E.; Robert, B.; Leconte, N.; Camier, B.; Rousseau, F.; Bourlieu, C.; Jardin, J.; Briard-Bion, V.; et al. Preparation and Characterisation of a Milk Polar Lipids Enriched Ingredient from Fresh Industrial Liquid Butter Serum: Combination of Physico-Chemical Modifications and Technological Treatments. Int. Dairy J. 2016, 52, 26–34. [Google Scholar] [CrossRef]
- Verardo, V.; Gómez-Caravaca, A.M.; Gori, A.; Losi, G.; Caboni, M.F. Bioactive Lipids in the Butter Production Chain from Parmigiano Reggiano Cheese Area. J. Sci. Food Agric. 2013, 93, 3625–3633. [Google Scholar] [CrossRef]
- Gallier, S.; Gragson, D.; Cabral, C.; Jiménez-Flores, R.; Everett, D.W. Composition and Fatty Acid Distribution of Bovine Milk Phospholipids from Processed Milk Products. J. Agric. Food Chem. 2010, 58, 10503–10511. [Google Scholar] [CrossRef]
- Fauquant, C.; Briard-Bion, V.; Leconte, N.; Guichardant, M.; Michalski, M.C. Membrane Phospholipids and Sterols in Microfiltered Milk Fat Globules. Eur. J. Lipid Sci. Technol. 2007, 109, 1167–1173. [Google Scholar] [CrossRef]
- Castro-Gómez, P.; Montero, O.; Fontecha, J. In-Depth Lipidomic Analysis of Molecular Species of Triacylglycerides, Diacylglycerides, Glycerophospholipids, and Sphingolipids of Buttermilk by GC-MS/FID, HPLC-ELSD, and UPLC-QTOF-MS. Int. J. Mol. Sci. 2017, 18, 605. [Google Scholar] [CrossRef] [PubMed]
- Jukkola, A.; Partanen, R.; Rojas, O.J.; Heino, A. Separation of Milk Fat Globules via Microfiltration: Effect of Diafiltration Media and Opportunities for Stream Valorization. J. Dairy Sci. 2016, 99, 8644–8654. [Google Scholar] [CrossRef]
- He, S.; Tang, H.; Yi, H.; Xu, W.; Ma, Y.; Wang, R. Properties of Emulsions from Milk Fat Globule Membrane and Its Components. Int. J. Food Prop. 2017, 20, 1342–1353. [Google Scholar] [CrossRef]
- Wiking, L.; Gregersen, S.B.; Hansen, S.F.; Hammershøj, M. Heat-Induced Changes in Milk Fat and Milk Fat Globules and Its Derived Effects on Acid Dairy Gelation—A Review. Int. Dairy J. 2022, 127, 105213. [Google Scholar] [CrossRef]
- Lee, S.J.; Sherbon, J.W. Chemical Changes in Bovine Milk Fat Globule Membrane Caused by Heat Treatment and Homogenization of Whole Milk. J. Dairy Res. 2002, 69, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, N.; Zhao, X.; Yang, J.; Zhang, Y.; Han, R.; Qi, Y.; Zhao, S.; Li, S.; Wen, F.; et al. Changes in Bovine Milk Fat Globule Membrane Proteins Caused by Heat Procedures Using a Label-Free Proteomic Approach. Food Res. Int. 2018, 113, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fong, B.Y.; Norris, C.S.; MacGibbon, A.K.H. Protein and Lipid Composition of Bovine Milk-Fat-Globule Membrane. Int. Dairy J. 2007, 17, 275–288. [Google Scholar] [CrossRef]
- Phan, T.T.Q.; Le, T.T.; Van der Meeren, P.; Dewettinck, K. Comparison of Emulsifying Properties of Milk Fat Globule Membrane Materials Isolated from Different Dairy By-Products. J. Dairy Sci. 2014, 97, 4799–4810. [Google Scholar] [CrossRef]
- Le, T.T.; Van Camp, J.; Dewettinck, K. Milk Fat Globule Membrane Material. In Studies in Natural Products Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 41, pp. 347–382. [Google Scholar] [CrossRef]
- Lu, J.; Argov-Argaman, N.; Anggrek, J.; Boeren, S.; van Hooijdonk, T.; Vervoort, J.; Hettinga, K.A. The Protein and Lipid Composition of the Membrane of Milk Fat Globules Depends on Their Size. J. Dairy Sci. 2016, 99, 4726–4738. [Google Scholar] [CrossRef]
- Bianchi, L.; Puglia, M.; Landi, C.; Matteoni, S.; Perini, D.; Armini, A.; Verani, M.; Trombetta, C.; Soldani, P.; Roncada, P.; et al. Solubilization Methods and Reference 2-DE Map of Cow Milk Fat Globules. J. Proteom. 2009, 72, 853–864. [Google Scholar] [CrossRef]
- Lu, J.; Boeren, S.; de Vries, S.C.; van Valenberg, H.J.F.; Vervoort, J.; Hettinga, K. Filter-Aided Sample Preparation with Dimethyl Labeling to Identify and Quantify Milk Fat Globule Membrane Proteins. J. Proteom. 2011, 75, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Cong, M.; Peng, X.; Wu, J.; Wu, R.; Liu, B.; Ye, W.; Yue, X. Quantitative Proteomic Analysis of Milk Fat Globule Membrane (MFGM) Proteins in Human and Bovine Colostrum and Mature Milk Samples through ITRAQ Labeling. Food Funct. 2016, 7, 2438–2450. [Google Scholar] [CrossRef]
- Hettinga, K.; van Valenberg, H.; de Vries, S.; Boeren, S.; van Hooijdonk, T.; van Arendonk, J.; Vervoort, J. The Host Defense Proteome of Human and Bovine Milk. PLoS ONE 2011, 6, e19433. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, C.; Sun, X.; Guo, M. Comparative Proteomics of Whey and Milk Fat Globule Membrane Proteins of Guanzhong Goat and Holstein Cow Mature Milk. J. Food Sci. 2019, 84, 244–253. [Google Scholar] [CrossRef]
- Thum, C.; Roy, N.C.; Everett, D.W.; McNabb, W.C. Variation in Milk Fat Globule Size and Composition: A Source of Bioactives for Human Health. Crit. Rev. Food Sci. Nutr. 2023, 63, 87–113. [Google Scholar] [CrossRef]
- Ye, A.; Singh, H.; Taylor, M.W.; Anema, S. Characterization of Protein Components of Natural and Heat-Treated Milk Fat Globule Membranes. Int. Dairy J. 2002, 12, 393–402. [Google Scholar] [CrossRef]
- He, S.; Ma, Y.; Wang, J.; Li, Q.; Tang, S.; Zhao, C.; Li, H.; Maubois, J.-L. Characterization of Fat Globules and Milk Fat Globule Membrane Proteins in Milk of Different Yak Breeds. Dairy Sci. Technol. 2010, 90, 601–609. [Google Scholar] [CrossRef]
- Haddadian, Z.; Eyres, G.T.; Carne, A.; Everett, D.W.; Bremer, P. Impact of Different Milk Fat Globule Membrane Preparations on Protein Composition, Xanthine Oxidase Activity, and Redox Potential. Int. Dairy J. 2017, 64, 14–21. [Google Scholar] [CrossRef]
- Singh, H.; Gallier, S. Nature’s Complex Emulsion: The Fat Globules of Milk. Food Hydrocoll. 2017, 68, 81–89. [Google Scholar] [CrossRef]
- Morin, P.; Jiménez-Flores, R.; Pouliot, Y. Effect of Processing on the Composition and Microstructure of Buttermilk and Its Milk Fat Globule Membranes. Int. Dairy J. 2007, 17, 1179–1187. [Google Scholar] [CrossRef]
- Hansen, S.F.; Hogan, S.A.; Tobin, J.; Rasmussen, J.T.; Larsen, L.B.; Wiking, L. Microfiltration of Raw Milk for Production of High-Purity Milk Fat Globule Membrane Material. J. Food Eng. 2020, 276, 109887. [Google Scholar] [CrossRef]
- Pallesen, L.T.; Pedersen, L.R.L.; Petersen, T.E.; Rasmussen, J.T. Characterization of Carbohydrate Structures of Bovine MUC15 and Distribution of the Mucin in Bovine Milk. J. Dairy Sci. 2007, 90, 3143–3152. [Google Scholar] [CrossRef]
- Le, T.T.; van Camp, J.; Rombaut, R.; van Leeckwyck, F.; Dewettinck, K. Effect of Washing Conditions on the Recovery of Milk Fat Globule Membrane Proteins during the Isolation of Milk Fat Globule Membrane from Milk. J. Dairy Sci. 2009, 92, 3592–3603. [Google Scholar] [CrossRef] [Green Version]
- Visioli, F. Science and Claims of the Arena of Food Bioactives: Comparison of Drugs, Nutrients, Supplements, and Nutraceuticals. Food Funct. 2022, 13, 12470–12474. [Google Scholar] [CrossRef]







| Sample | Fat | NL | PL | Protein |
|---|---|---|---|---|
| RWM | 29.63 ± 4.85 d | 29.63 ± 4.85 e | n.d. | 24.10 ± 0.62 de |
| PWM | 34.04 ± 0.76 e | 34.04 ± 0.77 f | n.d. | 19.43 ± 0.48 b |
| PSKM | 1.07 ± 0.52 a | 0.91 ± 0.43 a | 0.16 ± 0.09 a | 35.28 ± 0.74 i |
| RC | 88.69 ± 1.07 g | 88.69 ± 1.08 h | n.d. | 3.60 ± 0.25 a |
| PC | 85.38 ± 1.25 f | 85.38 ± 1.26 g | n.d. | 3.46 ± 0.36 a |
| BM1 | 12.18 ± 0.61 c | 8.80 ± 0.51 c | 3.38 ± 0.10 e | 21.07 ± 1.77 bc |
| BM2 | 6.91 ± 0.52 b | 6.52 ± 0.50 bc | 0.38 ± 0.02 b | 27.35 ± 1.98 g |
| BS1 | 6.18 ± 1.22 b | 5.66 ± 1.12 b | 0.36 ± 0.15 b | 29.56 ± 1.47 h |
| BS2 | 27.28 ± 0.44 d | 24.25 ± 0.37 d | 3.02 ± 0.07 d | 20.48 ± 0.42 b |
| BMP-1 | 12.28 ± 0.51 c | 8.91 ± 0.32 c | 3.37 ± 0.19 e | 22.52 ± 0.70 cd |
| BSP-1 | 5.51 ± 0.83 b | 5.08 ± 0.75 b | 0.43 ± 0.09 b | 25.24 ± 1.33 ef |
| BM-BS BLEND | 9.91 ± 1.24 c | 8.01 ± 1.14 bc | 1.91 ± 0.13 c | 26.84 ± 1.58 fg |
| FAME | RWM | PWM | PSKM | RC | PC | BM1 | BM2 | BS1 | BS2 | BMP-1 | BSP-1 | BM-BS BLEND |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C4:0 | 4.82 ± 1.18 abc | 4.57 ± 0.31 ab | 6.61 ± 1.41 cd | 5.65 ± 0.28 abc | 5.59 ± 0.94 abc | 4.30 ± 0.09 ab | 6.26 ± 0.15 bcd | 4.63 ± 0.28 abc | 5.12 ± 0.82 abc | 4.04 ± 0.56 a | 7.72 ± 2.71 d | 4.06 ± 0.42 a |
| C6:0 | 2.86 ± 0.60 e | 2.50 ± 0.24 de | 2.25 ± 0.48 cde | 2.68 ± 0.40 de | 2.73 ± 0.34 e | 1.30 ± 0.10 a | 2.77 ± 0.01 e | 1.65 ± 0.14 abc | 2.02 ± 0.02 bcd | 1.56 ± 0.14 ab | 2.29 ± 0.85 cde | 1.46 ± 0.11 ab |
| C8:0 | 2.09 ± 0.42 e | 1.86 ± 0.21 de | 1.45 ± 0.15 bc | 1.98 ± 0.30 de | 1.88 ± 0.14 de | 0.92 ± 0.10 a | 2.02 ± 0.18 e | 1.31 ± 0.03 abc | 1.60 ± 0.09 cd | 1.27 ± 0.15 abc | 1.57 ± 0.38 cd | 1.06 ± 0.12ab |
| C10:0 | 4.43 ± 0.68 ef | 4.11 ± 0.33 def | 3.22 ± 0.49 abc | 4.18 ± 0.46 ef | 4.22 ± 0.51 ef | 2.40 ± 0.03 a | 4.72 ± 0.29 f | 3.25 ± 0.30 abcd | 3.73 ± 0.23 bcde | 2.95 ± 0.28 ab | 4.08 ± 1.07 cdef | 2.66 ± 0.08 a |
| C12:0 | 4.61 ± 0.25 f | 4.48 ± 0.18 ef | 4.02 ± 0.23 de | 4.71 ± 0.25 f | 4.72 ± 0.35 f | 2.81 ± 0.01 a | 4.72 ± 0.02 f | 3.58 ± 0.08 cd | 3.91 ± 0.03 d | 3.35 ± 0.34 bc | 4.06 ± 0.62 de | 3.04 ± 0.06 ab |
| C14:0 | 12.21 ± 0.16 ef | 12.40 ± 0.01 f | 13.08 ± 0.54 g | 12.50 ± 0.29 f | 12.35 ± 0.02 f | 9.03 ± 0.01 a | 11.79 ± 0.14 de | 11.81 ± 0.19 de | 10.61 ± 0.34 c | 9.56 ± 0.47 b | 11.40 ± 0.39 d | 9.70 ± 0.15 b |
| C14:1 c9 | 1.15 ± 0.06 a | 1.15 ± 0.03 a | 1.13 ± 0.07 a | 1.18 ± 0.07 a | 1.30 ± 0.04 bc | 1.11 ± 0.04 a | 1.19 ± 0.07 ab | 1.31 ± 0.04 c | 1.08 ± 0.01 a | 1.14 ± 0.06 a | 1.30 ± 0.16 bc | 1.13 ± 0.03 a |
| C16:0 | 23.50 ± 0.74 c | 23.51 ± 0.44 c | 23.08 ± 0.13 c | 23.68 ± 1.29 c | 23.63 ± 0.54 c | 20.07 ± 0.02 a | 20.75 ± 0.08 ab | 24.74 ± 0.03 d | 21.07 ± 0.46 ab | 20.39 ± 0.25 ab | 21.52 ± 1.31 b | 20.95 ± 0.06 ab |
| C16:1 c9 | 1.15 ± 0.05 de | 1.13 ± 0.02 cde | 1.08 ± 0.08 bcd | 1.18 ± 0.04 e | 1.19 ± 0.01 e | 1.07 ± 0.01 abcd | 1.13 ± 0.06 cde | 0.99 ± 0.04 a | 1.06 ± 0.04 abc | 1.09 ± 0.04 bcd | 1.09 ± 0.09 bcd | 1.03 ± 0.02 ab |
| C18:0 | 6.70 ± 0.24 bcd | 6.99 ± 0.17 cd | 6.56 ± 0.59 bc | 6.67 ± 0.30 bc | 6.29 ± 0.23 ab | 7.69 ± 0.02 e | 5.96 ± 0.02 a | 7.91 ± 0.02 e | 6.95 ± 0.07 cd | 7.77 ± 0.09 e | 7.18 ± 0.55 d | 7.66 ± 0.08 e |
| C18:1 t10 | 0.24 ± 0.05 ab | 0.43 ± 0.08 c | 0.14 ± 0.14 a | 0.43 ± 0.07 c | 0.38 ± 0.06 c | 0.35 ± 0.06 bc | 0.24 ± 0.01 ab | 0.46 ± 0.01 c | 0.41 ± 0.01 c | 0.37 ± 0.11 c | 0.23 ± 0.04 a | 0.36 ± 0.01 c |
| C18:1 t11 (TVA) | 0.36 ± 0.06 a | 0.92 ± 0.31 b | 0.38 ± 0.38 a | 0.37 ± 0.37 a | 0.60 ± 0.10 ab | 0.54 ± 0.01 ab | 0.66 ± 0.06 ab | 0.53 ± 0.01 ab | 0.74 ± 0.16 ab | 0.63 ± 0.04 ab | 0.62 ± 0.26 ab | 0.61 ± 0.07 ab |
| C18:1 c9 | 24.70 ± 1.68 a | 24.20 ± 0.30 a | 24.54 ± 0.45 a | 23.32 ± 1.10 a | 23.17 ± 1.06 a | 30.22 ± 0.23 c | 23.36 ± 0.33 a | 24.43 ± 0.04 a | 26.66 ± 0.85 b | 29.65 ± 1.16 c | 23.14 ± 2.66 a | 29.06 ± 0.75 c |
| Total cis C18:1 | 25.84 ± 1.80 a | 25.47 ± 0.03 a | 24.99 ± 0.01 a | 24.54 ± 1.01 a | 24.53 ± 1.17 a | 32.38 ± 0.18 c | 24.99 ± 0.44 a | 25.90 ± 0.08 a | 28.33 ± 0.74 b | 30.65 ± 1.52 c | 24.22 ± 3.47 a | 30.75 ± 1.11 c |
| Total trans C18:1 | 1.96 ± 0.21 a | 2.86 ± 0.62 ab | 1.97 ± 1.43 a | 1.96 ± 0.77 a | 2.60 ± 0.20 ab | 2.88 ± 0.02 ab | 2.70 ± 0.05 ab | 2.66 ± 0.08 ab | 3.09 ± 0.55b | 2.38 ± 0.07 ab | 2.50 ± 0.69 ab | 2.75 ± 0.16 ab |
| C18:2 c9.c12 (LA) | 2.57 ± 0.19 a | 2.57 ± 0.12 a | 4.13 ± 0.36 cd | 2.49 ± 0.09 a | 2.41 ± 0.24 a | 6.85 ± 0.20 e | 3.50 ± 0.32 bc | 3.34 ± 1.05 b | 4.71 ± 0.02 d | 6.56 ± 0.66 e | 3.35 ± 0.22 b | 6.44 ± 0.04 e |
| C18:3 (ALA) | 0.40 ± 0.04 a | 0.54 ± 0.05 ab | 0.41 ± 0.41 a | 0.43 ± 0.08 a | 0.44 ± 0.02 ab | 0.61 ± 0.01 ab | 0.52 ± 0.08 ab | 0.46 ± 0.03 ab | 0.39 ± 0.09 a | 0.43 ± 0.16 a | 0.70 ± 0.06 b | 0.57 ± 0.03 ab |
| C18:2 c9.t11 (RA) | 0.70 ± 0.04 bcd | 0.91 ± 0.15 cd | 0.30 ± 0.31 a | 0.62 ± 0.01 bc | 0.52 ± 0.11 ab | 0.93 ± 0.10 d | 0.81 ± 0.26 cd | 0.90 ± 0.20 cd | 0.83 ± 0.02 cd | 1.24 ± 0.22 e | 0.72 ± 0.06 bcd | 0.88 ± 0.05 cd |
| C20:3 n6 (DGLA) | 0.09 ± 0.01 a | 0.08 ± 0.03 a | 0.03 ± 0.03 a | 0.01 ± 0.01 a | 0.07 ± 0.07 a | 0.71 ± 0.06 d | 0.44 ± 0.04 bc | 0.33 ± 0.07 b | 0.36 ± 0.12 b | 0.52 ± 0.12 c | 0.13 ± 0.13 a | 0.56 ± 0.04 c |
| C20:4 n6 (AA) | 0.11 ± 0.01 a | 0.10 ± 0.03 a | 0.15 ± 0.15 a | 0.06 ± 0.06 a | 0.13 ± 0.01 a | 0.66 ± 0.07 e | 0.45 ± 0.08 bc | 0.37 ± 0.05 b | 0.50 ± 0.03 cd | 0.67 ± 0.07 e | 0.07 ± 0.07 a | 0.60 ± 0.01 de |
| Total n3 | 0.40 ± 0.04 a | 0.54 ± 0.05 ab | 0.41 ± 0.41 a | 0.43 ± 0.08 a | 0.44 ± 0.02 ab | 0.61 ± 0.01 ab | 0.52 ± 0.08 ab | 0.46 ± 0.03 ab | 0.39 ± 0.09 a | 0.43 ± 0.16 a | 0.70 ± 0.06 b | 0.57 ± 0.03 ab |
| Total n6 | 2.78 ± 0.19 ab | 2.75 ± 0.17 ab | 4.31 ± 0.18 c | 2.56 ± 0.16 a | 2.61 ± 0.30 a | 8.21 ± 0.32 e | 4.39 ± 0.36 c | 4.03 ± 1.16 c | 5.58 ± 0.11 d | 7.76 ± 0.83 e | 3.55 ± 0.42 bc | 7.61 ± 0.09 e |
| n6/n3 | 6.98 ± 0.29 b | 5.16 ± 0.76 ab | 2.54 ± 2.54 a | 6.12 ± 0.66 ab | 5.91 ± 0.48 ab | 13.44 ± 0.76 c | 8.68 ± 1.95 b | 8.88 ± 3.06 b | 15.07 ± 2.96 c | 19.91 ± 5.37 d | 5.13 ± 1.05 ab | 13.42 ± 0.52 c |
| TAG (%) | RWM | PWM | PSKM | RC | PC | BM1 | BM2 | BS1 | BS2 | BMP-1 | BSP-1 | BM-BS BLEND |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CN24 | 0.08 ± 0.02 ab | 0.08 ± 0.03 ab | 0.50 ± 0.21 e | 0.05 ± 0.02 a | 0.05 ± 0.02 a | 0.31 ± 0.13 d | 0.17 ± 0.09 abcd | 0.29 ± 0.05 cd | 0.22 ± 0.01 bcd | 0.14 ± 0.03 abc | 0.24 ± 0.01 cd | 0.17 ± 0.06 abcd |
| CN26 | 0.33 ± 0.02 a | 0.32 ± 0.02 a | 1.08 ± 0.46 c | 0.27 ± 0.06 a | 0.28 ± 0.02 a | 0.48 ± 0.15 a | 0.55 ± 0.34 a | 0.55 ± 0.07 a | 0.67 ± 0.17 ab | 0.97 ± 0.27 bc | 0.63 ± 0.27 ab | 0.98 ± 0.05 bc |
| CN28 | 0.61 ± 0.01 c | 0.63 ± 0.04 c | 0.54 ± 0.03 b | 0.64 ± 0.01 c | 0.64 ± 0.01 c | 0.63 ± 0.01 c | 0.72 ± 0.04 d | 0.46 ± 0.03 a | 0.66 ± 0.06 c | 0.81 ± 0.01 e | 0.47 ± 0.06 a | 0.72 ± 0.02 d |
| CN30 | 1.32 ± 0.01 a | 1.35 ± 0.06 a | 2.14 ± 0.09 d | 1.33 ± 0.01 a | 1.36 ± 0.01 a | 1.66 ± 0.04 bc | 1.53 ± 0.07 ab | 1.29 ± 0.49 a | 1.66 ± 0.20 bc | 2.18 ± 0.01 d | 1.58 ± 0.07 ab | 1.92 ± 0.07 cd |
| CN32 | 2.73 ± 0.01 a | 2.76 ± 0.13 a | 4.53 ± 0.39 c | 2.73 ± 0.03 a | 2.79 ± 0.08 a | 3.47 ± 0.24 b | 3.67 ± 0.41 b | 3.34 ± 0.62 b | 3.30 ± 0.36 b | 4.57 ± 0.26 c | 3.66 ± 0.29 b | 4.25 ± 0.19 c |
| CN34 | 6.10 ± 0.05 a | 6.24 ± 0.16 a | 7.73 ± 0.34 bc | 6.17 ± 0.09 a | 6.22 ± 0.07 a | 6.49 ± 0.44 a | 7.41 ± 0.75 b | 7.85 ± 0.40 bc | 6.57 ± 0.74 a | 8.27 ± 0.19 c | 7.43 ± 0.10 b | 8.11 ± 0.19 bc |
| CN36 | 11.11 ± 0.05 abc | 11.37 ± 0.24 abc | 13.07 ± 0.21 de | 11.22 ± 0.03 abc | 11.08 ± 0.19 abc | 10.98 ± 0.81 ab | 10.69 ± 0.72 a | 11.06 ± 0.54 abc | 12.31 ± 1.78 cde | 14.88 ± 0.46 f | 12.01 ± 0.25 bcd | 13.36 ± 0.68 e |
| CN38 | 12.85 ± 0.05 d | 13.07 ± 0.36 d | 10.14 ± 0.22 b | 13.16 ± 0.06 d | 12.91 ± 0.13 d | 9.68 ± 0.48 ab | 11.70 ± 1.11 c | 9.11 ± 0.52 a | 11.42 ± 0.82c | 9.00 ± 0.63 a | 9.64 ± 0.33 ab | 10.08 ± 0.27 b |
| CN40 | 10.10 ± 0.05 ab | 10.20 ± 0.10 ab | 11.02 ± 0.23 b | 10.24 ± 0.25 ab | 10.15 ± 0.13 ab | 19.09 ± 0.40 e | 10.62 ± 0.99 ab | 10.57 ± 0.24 ab | 12.69 ± 1.35 c | 15.68 ± 0.84 d | 9.48 ± 0.40 a | 12.34 ± 0.53 c |
| CN42 | 7.38 ± 0.05 e | 7.18 ± 0.02 de | 7.09 ± 0.15 cde | 7.40 ± 0.09 e | 7.49 ± 0.08 e | 6.42 ± 0.44 b | 6.68 ± 0.08 bc | 6.72 ± 0.59 bc | 6.90 ± 0.22 cd | 5.61 ± 0.08 a | 6.35 ± 0.10 b | 5.73 ± 0.22 a |
| CN44 | 7.03 ± 0.09 f | 6.78 ± 0.12 ef | 7.47 ± 0.27 g | 6.99 ± 0.10 f | 7.05 ± 0.09 f | 5.68 ± 0.24 a | 6.12 ± 0.38 bc | 6.97 ± 0.15 f | 6.38 ± 0.04 cd | 5.63 ± 0.06 a | 6.53 ± 0.15 de | 5.92 ± 0.14 ab |
| CN46 | 7.48 ± 0.15 fgh | 7.29 ± 0.11 ef | 8.05 ± 0.11 i | 7.37 ± 0.09 fg | 7.43 ± 0.20 fg | 6.06 ± 0.22 a | 6.98 ± 0.37 de | 7.79 ± 0.17 hi | 6.69 ± 0.22 cd | 6.32 ± 0.06 ab | 7.67 ± 0.26 gh | 6.59 ± 0.09 bc |
| CN48 | 9.35 ± 0.12 d | 8.98 ± 0.11 cd | 8.89 ± 0.18 cd | 9.20 ± 0.12 d | 9.23 ± 0.19 d | 7.52 ± 0.40 a | 8.97 ± 0.51 cd | 10.50 ± 0.31 e | 8.59 ± 0.55 bc | 7.64 ± 0.18 a | 10.35 ± 0.20 e | 8.29 ± 0.07 b |
| CN50 | 10.92 ± 0.21 efg | 10.81± 0.30 defg | 9.48 ± 0.18 b | 10.76 ± 0.18 def | 10.90 ± 0.16 efg | 9.99 ± 0.28 bcd | 10.28 ±0.58 bcde | 11.65 ± 0.59 g | 10.38 ±1.04 cdef | 8.12 ± 0.60 a | 11.21 ± 0.10 fg | 9.71 ± 0.09 bc |
| CN52 | 8.84 ± 0.08 def | 8.90 ± 0.20 def | 6.35 ± 0.32 a | 8.91 ± 0.20 def | 8.76 ± 0.10 def | 8.05 ± 1.15 cd | 9.42 ± 0.81 f | 8.38 ± 0.41 cde | 7.83 ± 0.23 bc | 7.15 ± 0.38 b | 9.13 ± 0.19 ef | 8.34 ± 0.11 cde |
| CN54 | 3.76 ± 0.13 bcd | 4.03 ± 0.38 cd | 1.90 ± 0.23 a | 3.55 ± 0.32 bc | 3.67 ± 0.29 bc | 3.51 ± 0.66 bc | 4.49 ± 0.62 d | 3.49 ± 0.48 bc | 3.74 ± 0.74 bcd | 3.02 ± 0.16 b | 3.60 ± 0.27 bc | 3.48 ± 0.11 bc |
| Lipid Classes | BM1 | MFGM BM1 | BM2 | MFGM BM2 | BS2 | MFGM BS2 | BMP-1 | MFGM BMP-1 | BM-BS BLEND | MFGM BM-BS BLEND |
|---|---|---|---|---|---|---|---|---|---|---|
| TAG | 58.20 ± 0.80 a | 25.84 ± 1.50 b | 78.59 ± 6.68 a | 29.26 ± 0.86 b | 85.34 ± 0.26 a | 47.45 ± 0.73 b | 63.10 ± 0.93 a | 40.02 ± 0.87 b | 63.61 ± 3.02 a | 37.83 ± 2.27 b |
| DAG | 9.76 ± 0.56 | 12.38 ± 0.45 | 13.30 ± 5.63 a | 23.56 ± 0.96 b | 2.70 ± 0.28 a | 7.43 ± 0.92 b | 6.72 ± 0.49 | 8.51 ± 0.30 | 12.58 ± 1.59 a | 17.21 ± 2.09 b |
| FFA CHOL | 3.02 ± 0.24 | 3.38 ± 0.39 | 2.30 ± 0.97 a | 8.82 ± 0.67 b | 0.48 ± 0.02 a | 1.40 ± 0.21 b | 1.64 ± 0.06 | 1.58 ± 0.18 | 1.82 ± 0.14 | 2.43 ± 0.50 |
| MAG | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.03 a | 0.32 ± 0.09 b | 0.03 ± 0.00 | 0.07 ± 0.02 | 0.05 ± 0.02 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.03 |
| GLUCER | 0.40 ± 0.08 a | 1.00 ± 0.03 b | 0.07 ± 0.01 a | 0.57 ± 0.01 b | 0.14 ± 0.02 a | 0.69 ± 0.07 b | 0.36 ± 0.02 a | 0.72 ± 0.10 b | 0.30 ± 0.07 a | 0.81 ± 0.05 b |
| LACER | 0.75 ± 0.05 a | 1.56 ± 0.06 b | 0.08 ± 0.00 a | 0.67 ± 0.14 b | 0.23 ± 0.00 a | 1.02 ± 0.12 b | 0.70 ± 0.04 a | 1.19 ± 0.13 b | 0.42 ± 0.15 a | 1.14 ± 0.05 b |
| PL | 27.79 ± 0.56 a | 55.69 ± 0.78 b | 5.57 ± 0.15 a | 36.69 ± 1.25 b | 11.08 ± 0.08 a | 41.60 ± 0.44 b | 27.42 ± 0.43 a | 47.66 ± 0.76 b | 19.33 ± 1.57 a | 40.35 ± 0.48 b |
| NL | 72.21 ± 0.56 a | 44.31 ± 0.78 b | 94.43 ± 0.15 a | 63.31 ± 1.25 b | 88.92 ± 0.08 a | 58.40 ± 0.44 b | 72.58 ± 0.43 a | 52.34 ± 0.76 b | 80.67 ± 1.57 a | 59.65 ± 0.48 b |
| Band | Tryptic Peptide (Ion) | Protein Fragment | Sequence | Ion Score | Protein Name | Entry Number | Mascot Score |
|---|---|---|---|---|---|---|---|
| A | 1216.84 | 949–958 | K.EGDLTHFNQR.L | 50 | XDH | P80457 | 70 |
| A | 1334.96 | 1229–1240 | K.IPAFGSIPTEFR.V | 35 | XDH | P80457 | 70 |
| D | 961.87 | 70–78 | K.VSPAVFVSR.E | 47 | BTN | P18892 | 131 |
| D | 1018.94 | 411–420 | R.TPLPLAGPPR.R | 38 | BTN | P18892 | 131 |
| D | 1077.85 | 368–376 | R.TDWAIGVCR.E + Carbamidomethyl (C) | 27 | BTN | P18892 | 131 |
| D | 1124.97 | 332–340 | R.QKLPEKPER.F | 27 | BTN | P18892 | 131 |
| D | 1254.8782 | 341–350 | R.FDSWPCVMGR.E + Carbamidomethyl (C) | 40 | BTN | P18892 | 131 |
| D | 1255.8504 | 193–203 | R.NPDEEGLFTVR.A | 35 | BTN | P18892 | 131 |
| D | 1276.9567 | 358–367 | R.HYWEVEVGDR.T | 46 | BTN | P18892 | 131 |
| E | 1121.1414 | 409–417 | R.IQPVAWHNR.I | 35 | PAS 6 | Q95114 | 127 |
| E | 1194.1710 | 207–216 | R.QFQFIQVAGR.S | 61 | PAS 6 | Q95114 | 127 |
| E | 1223.1538 | 396–405 | K.NIFETPFQAR.F | 32 | PAS 6 | Q95114 | 127 |
| E | 1440.2549 | 349–360 | R.DFGHIQYVAAYR.V | 75 | PAS 6 | Q95114 | 127 |
| F | 842.7880 | 140–146 | R.WAPELAR.L | 27 | PAS 7 | Q95114 | 132 |
| F | 1007.9321 | 409–417 | R.LVPIICHR.G + Carbamidomethyl (C) | 16 | PAS 7 | Q95114 | 132 |
| F | 1120.9565 | 207–216 | R.IQPVAWHNR.I | 48 | PAS 7 | Q95114 | 132 |
| F | 1193.9977 | 396–405 | R.QFQFIQVAGR.S | 78 | PAS 7 | Q95114 | 132 |
| F | 1222.9794 | 175–187 | K.NIFETPFQAR.F | 34 | PAS 7 | Q95114 | 132 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Señoráns, M.; Gallo, V.; Calvo, M.V.; Fontecha, J. Lipidomic and Proteomic Profiling of the Milk Fat Globule Membrane from Different Industrial By-Products of the Butter and Butter Oil Manufacturing Process. Foods 2023, 12, 750. https://doi.org/10.3390/foods12040750
Señoráns M, Gallo V, Calvo MV, Fontecha J. Lipidomic and Proteomic Profiling of the Milk Fat Globule Membrane from Different Industrial By-Products of the Butter and Butter Oil Manufacturing Process. Foods. 2023; 12(4):750. https://doi.org/10.3390/foods12040750
Chicago/Turabian StyleSeñoráns, María, Veronica Gallo, María V. Calvo, and Javier Fontecha. 2023. "Lipidomic and Proteomic Profiling of the Milk Fat Globule Membrane from Different Industrial By-Products of the Butter and Butter Oil Manufacturing Process" Foods 12, no. 4: 750. https://doi.org/10.3390/foods12040750