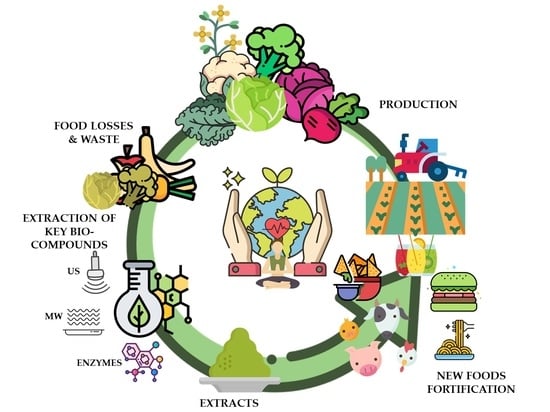
Genus Brassica By-Products Revalorization with Green Technologies to Fortify Innovative Foods: A Scoping Review
Abstract
:1. Background
2. Brassica By-Products and Their Added-Value Compounds
3. Materials and Methods
4. Abiotic Stresses to Enhance Bioactive Compounds in Brassica By-Products
4.1. Ultraviolet Radiation
4.2. Wounding/Cutting
5. Extraction Techniques
5.1. Ultrasound-Assisted Extraction from Brassica By-Products
5.2. Microwave-Assisted Extraction from Brassica By-Products
5.3. Enzymatic-Assisted Extraction from Brassica By-Products
5.4. Other Extraction Methods from Brassica By-Products
6. Brassica By-Products Fortification in Food Matrices
6.1. Brassica By-Products Processing Pretreatments
6.2. Brassica By-Product Fortification in Animal Feed to Increase Functionality
6.3. Brassica By-Products Fortification in Several Food Matrices
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Food Loss and Waste Database. 2022. Available online: https://www.fao.org/platform-food-loss-waste/flw-data/en/ (accessed on 20 July 2022).
- European Union Food Loss and Waste Prevention. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy/food-loss-and-waste-prevention_en (accessed on 5 December 2022).
- UNEP Food Waste Index Report 2021. Available online: https://www.unep.org/resources/report/unep-food-waste-index-report-2021 (accessed on 5 December 2022).
- Stenmarck, A.; Jensen, C.; Quested, T.; Moates, G. Estimates of European Food Waste Levels. Available online: http://www.eu-fusions.org/phocadownload/Publications/Estimates%20of%20European%20food%20waste%20levels.pdf (accessed on 5 December 2022).
- Caldeira, C.; de Laurentiis, V.; Corrado, S.; van Holsteijn, F.; Sala, S. Quantification of Food Waste per Product Group along the Food Supply Chain in the European Union: A Mass Flow Analysis. Resour. Conserv. Recycl. 2019, 149, 479–488. [Google Scholar] [CrossRef]
- Corrado, S.; Ardente, F.; Sala, S.; Saouter, E. Modelling of Food Loss within Life Cycle Assessment: From Current Practice towards a Systematisation. J. Clean. Prod. 2017, 140, 847–859. [Google Scholar] [CrossRef]
- Caldeira, C.; Vlysidis, A.; Fiore, G.; de Laurentiis, V.; Vignali, G.; Sala, S. Sustainability of Food Waste Biorefinery: A Review on Valorisation Pathways, Techno-Economic Constraints, and Environmental Assessment. Bioresour. Technol. 2020, 312, 123575. [Google Scholar] [CrossRef] [PubMed]
- European Commission. A Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System. In Communication from The Commission to the European Parliament, the Council, the European Economic and Social Committee and The Committee of the Regions; European Commission: Brussels, Belgium, 2020; Volume 381. [Google Scholar]
- de Tamokou, J.D.; Mbaveng, A.T.; Kuete, V. Antimicrobial Activities of African Medicinal Spices and Vegetables. In Medicinal Spices and Vegetables from Africa: Therapeutic Potential Against Metabolic, Inflammatory, Infectious and Systemic Diseases; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 207–237. ISBN 9780128094419. [Google Scholar]
- Salehi, B.; Quispe, C.; Butnariu, M.; Sarac, I.; Marmouzi, I.; Kamle, M.; Tripathi, V.; Kumar, P.; Bouyahya, A.; Capanoglu, E.; et al. Phytotherapy and Food Applications from Brassica Genus. Phytother. Res. 2021, 35, 3590–3609. [Google Scholar] [CrossRef] [PubMed]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Effects of UV-B and UV-C Combination on Phenolic Compounds Biosynthesis in Fresh-Cut Carrots. Postharvest Biol. Technol. 2017, 127, 99–104. [Google Scholar] [CrossRef]
- Castillejo, N.; Martínez-Hernández, G.B.; Artés-Hernández, F. Revalorized Broccoli By-Products and Mustard Improved Quality during Shelf Life of a Kale Pesto Sauce. Food Sci. Technol. Int. 2021, 27, 734–745. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef]
- Kamal, M.M.; Akter, S.; Lin, C.N.; Nazzal, S. Sulforaphane as an Anticancer Molecule: Mechanisms of Action, Synergistic Effects, Enhancement of Drug Safety, and Delivery Systems. Arch. Pharm. Res. 2020, 43, 371–384. [Google Scholar] [CrossRef]
- Ho, E.; Clarke, J.D.; Dashwood, R.H. Dietary Sulforaphane, a Histone Deacetylase Inhibitor for Cancer Prevention. J. Nutr. 2009, 139, 2393–2396. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zheng, S.; Liu, Y.; Fang, Z.; Yang, L.; Zhuang, M.; Zhang, Y.; Lv, H.; Wang, Y.; Xu, D. Characterization of Glucosinolates in 80 Broccoli Genotypes and Different Organs Using UHPLC-Triple-TOF-MS Method. Food Chem. 2021, 334, 127519. [Google Scholar] [CrossRef]
- Hahn, C.; Müller, A.; Kuhnert, N.; Albach, D. Diversity of Kale (Brassica Oleracea Var. Sabellica): Glucosinolate Content and Phylogenetic Relationships. J. Agric. Food Chem. 2016, 64, 3215–3225. [Google Scholar] [CrossRef]
- Pongmalai, P.; Devahastin, S.; Chiewchan, N.; Soponronnarit, S. Enhancing the Recovery of Cabbage Glucoraphanin through the Monitoring of Sulforaphane Content and Myrosinase Activity during Extraction by Different Methods. Sep. Purif. Technol. 2017, 174, 338–344. [Google Scholar] [CrossRef]
- Drozdowska, M.; Leszczyńska, T.; Koronowicz, A.; Piasna-Słupecka, E.; Dziadek, K. Comparative Study of Young Shoots and the Mature Red Headed Cabbage as Antioxidant Food Resources with Antiproliferative Effect on Prostate Cancer Cells. RSC Adv. 2020, 10, 43021–43034. [Google Scholar] [CrossRef]
- Guijarro-Real, C.; Hernández-Cánovas, L.; Abellán-Victorio, Á.; Ben-Romdhane, O.; Moreno, D.A. The Combination of Monochromatic LEDs and Elicitation with Stressors Enhances the Accumulation of Glucosinolates in Mustard Sprouts with Species-Dependency. Plants 2022, 11, 2961. [Google Scholar] [CrossRef]
- Yi, G.; Lim, S.; Chae, W.B.; Park, J.E.; Park, H.R.; Lee, E.J.; Huh, J.H. Root Glucosinolate Profiles for Screening of Radish (Raphanus Sativus L.) Genetic Resources. J. Agric. Food Chem. 2016, 64, 61–70. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, Y.; Wang, X.; Yue, Z.; Yang, X.; Chen, X.; Zhang, X.; Shen, D.; Wang, H.; Song, J.; et al. Insights into the Species-Specific Metabolic Engineering of Glucosinolates in Radish (Raphanus Sativus L.) Based on Comparative Genomic Analysis. Sci. Rep. 2017, 7, 16040. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Li, Y.; Bao, T.; Zheng, X.; Chen, W.; Wang, J. A Recyclable Protein Resource Derived from Cauliflower By-Products: Potential Biological Activities of Protein Hydrolysates. Food Chem. 2017, 221, 114–122. [Google Scholar] [CrossRef]
- Gudiño, I.; Martín, A.; Casquete, R.; Prieto, M.H.; Ayuso, M.C.; Córdoba, M.G. Evaluation of Broccoli (Brassica Oleracea Var. Italica) Crop by-Products as Sources of Bioactive Compounds. Sci. Hortic. 2022, 304, 111284. [Google Scholar] [CrossRef]
- Thomas, M.; Badr, A.; Desjardins, Y.; Gosselin, A.; Angers, P. Characterization of Industrial Broccoli Discards (Brassica Oleracea Var. Italica) for Their Glucosinolate, Polyphenol and Flavonoid Contents Using UPLC MS/MS and Spectrophotometric Methods. Food Chem. 2018, 245, 1204–1211. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Ser, S.L.; Cumming, J.R.; Ku, K.-M. Comparative Phytonutrient Analysis of Broccoli By-Products: The Potentials for Broccoli By-Product Utilization. Molecules 2018, 23, 900. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; García-Viguera, C.; Moreno, D.A. Broccoli-Derived by-Products—A Promising Source of Bioactive Ingredients. J. Food Sci. 2010, 75, C383–C392. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Zevallos, L. The Use of Controlled Postharvest Abiotic Stresses as a Tool for Enhancing the Nutraceutical Content and Adding-Value of Fresh Fruits and Vegetables. J. Food Sci. 2003, 68, 1560–1565. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Miranda-Molina, F.D.; Klug, T.V.; Martínez-Hernández, G.B. Enrichment of Glucosinolate and Carotenoid Contents of Mustard Sprouts by Using Green Elicitors during Germination. J. Food Compos. Anal. 2022, 110, 104546. [Google Scholar] [CrossRef]
- Topcu, Y.; Dogan, A.; Kasimoglu, Z.; Sahin-Nadeem, H.; Polat, E.; Erkan, M. The Effects of UV Radiation during the Vegetative Period on Antioxidant Compounds and Postharvest Quality of Broccoli (Brassica Oleracea L.). Plant Physiol. Biochem. 2015, 93, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Castillejo, N.; Martínez-Zamora, L.; Artés-Hernández, F. Periodical UV-B Radiation Hormesis in Biosynthesis of Kale Sprouts Nutraceuticals. Plant Physiol. Biochem. 2021, 165, 274–285. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. Postharvest UV-B and UV-C Radiation Enhanced the Biosynthesis of Glucosinolates and Isothiocyanates in Brassicaceae Sprouts. Postharvest Biol. Technol. 2021, 181, 111650. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Gómez, P.A.; Pradas, I.; Artés, F.; Artés-Hernández, F. Moderate UV-C Pretreatment as a Quality Enhancement Tool in Fresh-Cut Bimi® Broccoli. Postharvest Biol. Technol. 2011, 62, 327–337. [Google Scholar] [CrossRef]
- Tomás-Callejas, A.; Otón, M.; Artés, F.; Artés-Hernández, F. Combined Effect of UV-C Pretreatment and High Oxygen Packaging for Keeping the Quality of Fresh-Cut Tatsoi Baby Leaves. Innov. Food Sci. Emerg. Technol. 2012, 14, 115–121. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Artés-Hernández, F.; Gómez, P.A.; Formica, A.C.; Artés, F. Combination of Electrolysed Water, UV-C and Superatmospheric O2 Packaging for Improving Fresh-Cut Broccoli Quality. Postharvest Biol. Technol. 2013, 76, 125–134. [Google Scholar] [CrossRef]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Use of Postharvest UV-B and UV-C Radiation Treatments to Revalorize Broccoli Byproducts and Edible Florets. Innov. Food Sci. Emerg. Technol. 2017, 43, 77–83. [Google Scholar] [CrossRef]
- Seljåsen, R.; Kusznierewicz, B.; Bartoszek, A.; Mølmann, J.; Vågen, I.M. Effects of Post-Harvest Elicitor Treatments with Ultrasound, UV- and Photosynthetic Active Radiation on Polyphenols, Glucosinolates and Antioxidant Activity in a Waste Fraction of White Cabbage (Brassica Oleracea Var. Capitata). Molecules 2022, 27, 5256. [Google Scholar] [CrossRef] [PubMed]
- Surjadinata, B.B.; Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. UVA, UVB and UVC Light Enhances the Biosynthesis of Phenolic Antioxidants in Fresh-Cut Carrot through a Synergistic Effect with Wounding. Molecules 2017, 22, 668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, Y.; Hu, W.; Jiang, A.; Xu, Y.; Zhao, M.; Yu, J.; Ji, Y.; Sarengaowa, Y.J.; Yang, X.; Feng, K. The Effect of Cutting Style on the Biosynthesis of Phenolics and Cellular Antioxidant Capacity in Wounded Broccoli. Food Res. Int. 2020, 137, 109565. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-García, D.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as Biofactories: Postharvest Stress-Induced Accumulation of Phenolic Compounds and Glucosinolates in Broccoli Subjected to Wounding Stress and Exogenous Phytohormones. Front. Plant Sci. 2016, 7, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana-Gálvez, J.; Santacruz, A.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Postharvest Wounding Stress in Horticultural Crops as a Tool for Designing Novel Functional Foods and Beverages with Enhanced Nutraceutical Content: Carrot Juice as a Case Study. J. Food Sci. 2019, 84, 1151–1161. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Artés-Hernández, F. By-Products Revalorization with Non-Thermal Treatments to Enhance Phytochemical Compounds of Fruit and Vegetables Derived Products: A Review. Foods 2022, 11, 59. [Google Scholar] [CrossRef]
- Pezeshkpour, V.; Khosravani, S.A.; Ghaedi, M.; Dashtian, K.; Zare, F.; Sharifi, A.; Jannesar, R.; Zoladl, M. Ultrasound Assisted Extraction of Phenolic Acids from Broccoli Vegetable and Using Sonochemistry for Preparation of MOF-5 Nanocubes: Comparative Study Based on Micro-Dilution Broth and Plate Count Method for Synergism Antibacterial Effect. Ultrason. Sonochem. 2018, 40, 1031–1038. [Google Scholar] [CrossRef]
- Yagoub, A.A.; Ma, H.; Zhou, C. Ultrasonic-Assisted Extraction of Protein from Rapeseed (Brassica Napus L.) Meal: Optimization of Extraction Conditions and Structural Characteristics of the Protein. Int. Food Res. J. 2017, 24, 621–629. [Google Scholar]
- Oniszczuk, A.; Olech, M. Optimization of Ultrasound-Assisted Extraction and LC-ESI-MS/MS Analysis of Phenolic Acids from Brassica Oleracea L. Var. Sabellica. Ind. Crops Prod. 2016, 83, 359–363. [Google Scholar] [CrossRef]
- Prá, V.D.; Dolwitsch, C.B.; Lima, F.O.; de Carvalho, C.A.; Viana, C.; do Nascimento, P.C.; da Rosa, M.B. Ultrasound-Assisted Extraction and Biological Activities of Extracts of Brassica Oleracea Var. Capitata. Food Technol. Biotechnol. 2015, 53, 102–109. [Google Scholar] [CrossRef]
- Pagliari, S.; Giustra, C.M.; Magoni, C.; Celano, R.; Fusi, P.; Forcella, M.; Sacco, G.; Panzeri, D.; Campone, L.; Labra, M. Optimization of Ultrasound-Assisted Extraction of Naturally Occurring Glucosinolates from by-Products of Camelina Sativa L. and Their Effect on Human Colorectal Cancer Cell Line. Front. Nutr. 2022, 9, 901944. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Li, X.; Xiao, J.; Guo, L. Enhancement of Ultrasound-Assisted Extraction of Sulforaphane from Broccoli Seeds via the Application of Microwave Pretreatment. Ultrason. Sonochem. 2022, 87, 106061. [Google Scholar] [CrossRef]
- Li, W.; Gong, P.; Ma, H.; Xie, R.; Wei, J.; Xu, M. Ultrasound Treatment Degrades, Changes the Color, and Improves the Antioxidant Activity of the Anthocyanins in Red Radish. LWT 2022, 165, 113761. [Google Scholar] [CrossRef]
- Mahn, A.; Quintero, J.; Castillo, N.; Comett, R. Effect of Ultrasound-Assisted Blanching on Myrosinase Activity and Sulforaphane Content in Broccoli Florets. Catalysts 2020, 10, 616. [Google Scholar] [CrossRef]
- Major, N.; Prekalj, B.; Perković, J.; Ban, D.; Užila, Z.; Ban, S.G. The Effect of Different Extraction Protocols on Brassica Oleracea Var. Acephala Antioxidant Activity, Bioactive Compounds, and Sugar Profile. Plants 2020, 9, 1792. [Google Scholar] [CrossRef]
- Amofa-Diatuo, T.; Anang, D.M.; Barba, F.J.; Tiwari, B.K. Development of New Apple Beverages Rich in Isothiocyanates by Using Extracts Obtained from Ultrasound-Treated Cauliflower by-Products: Evaluation of Physical Properties and Consumer Acceptance. J. Food Compos. Anal. 2017, 61, 73–81. [Google Scholar] [CrossRef]
- Marinelli, V.; Spinelli, S.; Angiolillo, L.; del Nobile, M.A.; Conte, A. Emerging Techniques Applied to By-Products for Food Fortification. J. Food Sci. Technol. 2020, 57, 905–914. [Google Scholar] [CrossRef]
- Stevanato, N.; da Silva, C. Radish Seed Oil: Ultrasound-Assisted Extraction Using Ethanol as Solvent and Assessment of Its Potential for Ester Production. Ind. Crops Prod. 2019, 132, 283–291. [Google Scholar] [CrossRef]
- Rivas, M.Á.; Benito, M.J.; Martín, A.; de Córdoba, M.G.; Ruíz-Moyano, S.; Casquete, R. Improve the Functional Properties of Dietary Fibre Isolated from Broccoli By-Products by Using Different Technologies. Innov. Food Sci. Emerg. Technol. 2022, 80, 103075. [Google Scholar] [CrossRef]
- Angiolillo, L.; Spinelli, S.; Marinelli, V.; Conte, A.; Nobile, M.A. del Extract from Broccoli By-Products to Extend the Shelf Life of Fish Burgers. J. Food Res. 2019, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Khajeh, M.; Akbarian, M.A.; Ghaffari-Moghaddam, M.; Bohlooli, M. Use of Response Surface Methodology in the Optimization of the Microwave Assisted Extraction Method for Determination of Multielements in Brassica Oleracea Var. Capitata (Cabbage) Samples. J. Food Meas. Charact. 2015, 9, 550–556. [Google Scholar] [CrossRef]
- Lin, Y.; Pi, J.; Jin, P.; Liu, Y.; Mai, X.; Li, P.; Fan, H. Enzyme and Microwave Co-Assisted Extraction, Structural Characterization and Antioxidant Activity of Polysaccharides from Purple-Heart Radish. Food Chem. 2022, 372, 131274. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, E.H.; Liakopoulou-Kyriakides, M. Agro-Food Wastes Utilization by Blakeslea Trispora for Carotenoids Production. Acta Biochim. Pol. 2012, 59, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Arfat, Y.; Aziz, R.S.; Ali, L.; Ahmed, H.; Asim, S.; Rashid, M.; Hocart, C.H. Enzymatically Assisted Extraction of Antioxidant and Anti-Mutagenic Compounds from Radish (Raphanus Sativus). Environ. Technol. Innov. 2021, 23, 101620. [Google Scholar] [CrossRef]
- Tanongkankit, Y.; Sablani, S.S.; Chiewchan, N.; Devahastin, S. Microwave-Assisted Extraction of Sulforaphane from White Cabbages: Effects of Extraction Condition, Solvent and Sample Pretreatment. J. Food Eng. 2013, 117, 151–157. [Google Scholar] [CrossRef]
- García, S.L.R.; Raghavan, V. Microwave-Assisted Extraction of Phenolic Compounds from Broccoli (Brassica Oleracea) Stems, Leaves, and Florets: Optimization, Characterization, and Comparison with Maceration Extraction. Recent Prog. Nutr. 2022, 2, 1. [Google Scholar] [CrossRef]
- Tian, Y.; Kriisa, M.; Föste, M.; Kütt, M.L.; Zhou, Y.; Laaksonen, O.; Yang, B. Impact of Enzymatic Pre-Treatment on Composition of Nutrients and Phytochemicals of Canola (Brassica Napus) Oil Press Residues. Food Chem. 2022, 387, 132911. [Google Scholar] [CrossRef]
- Zykwinska, A.; Boiffard, M.H.; Kontkanen, H.; Buchert, J.; Thibault, J.F.; Bonnin, E. Extraction of Green Labeled Pectins and Pectic Oligosaccharides from Plant Byproducts. J. Agric. Food Chem. 2008, 56, 8926–8935. [Google Scholar] [CrossRef]
- Petkowicz, C.L.O.; Williams, P.A. Pectins from Food Waste: Characterization and Functional Properties of a Pectin Extracted from Broccoli Stalk. Food Hydrocoll. 2020, 107, 105930. [Google Scholar] [CrossRef]
- González, F.; Quintero, J.; del Río, R.; Mahn, A. Optimization of an Extraction Process to Obtain a Food-Grade Sulforaphane-Rich Extract from Broccoli (Brassica Oleracea Var. Italica). Molecules 2021, 26, 4042. [Google Scholar] [CrossRef]
- Shi, M.; Ying, D.Y.; Hlaing, M.M.; Ye, J.H.; Sanguansri, L.; Augustin, M.A. Development of Broccoli By-Products as Carriers for Delivering EGCG. Food Chem. 2019, 301, 125301. [Google Scholar] [CrossRef]
- Sinichi, S.; Siañez, A.V.L.; Diosady, L.L. Recovery of Phenolic Compounds from the By-Products of Yellow Mustard Protein Isolation. Food Res. Int. 2019, 115, 460–466. [Google Scholar] [CrossRef]
- Borja-Martínez, M.; Lozano-Sánchez, J.; Borrás-Linares, I.; Pedreño, M.A.; Sabater-Jara, A.B. Revalorization of Broccoli By-Products for Cosmetic Uses Using Supercritical Fluid Extraction. Antioxidants 2020, 9, 1195. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. From Pomegranate Byproducts Waste to Worth: A Review of Extraction Techniques and Potential Applications for Their Revalorization. Foods 2022, 11, 2596. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Monteiro, F.; Passos, C.P.; Silva, A.M.S.; Wessel, D.F.; Coimbra, M.A.; Cardoso, S.M. Blanching Impact on Pigments, Glucosinolates, and Phenolics of Dehydrated Broccoli by-Products. Food Res. Int. 2020, 132, 109055. [Google Scholar] [CrossRef]
- Tanongkankit, Y.; Chiewchan, N.; Devahastin, S. Effect of Processing on Antioxidants and Their Activity in Dietary Fiber Powder from Cabbage Outer Leaves. Dry. Technol. 2010, 28, 1063–1071. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Cardoso, S.M.; Wessel, D.F.; Coimbra, M.A. Microwave Assisted Dehydration of Broccoli By-Products and Simultaneous Extraction of Bioactive Compounds. Food Chem. 2018, 246, 386–393. [Google Scholar] [CrossRef]
- Khukutapan, D.; Chiewchan, N.; Devahastin, S. Characterization of Nanofibrillated Cellulose Produced by Different Methods from Cabbage Outer Leaves. J. Food Sci. 2018, 83, 1660–1667. [Google Scholar] [CrossRef]
- Betoret, E.; Rosell, C.M. Effect of Particle Size on Functional Properties of Brassica Napobrassica Leaves Powder. Starch Interactions and Processing Impact. Food Chem. X 2020, 8, 100106. [Google Scholar] [CrossRef]
- Martins, T.; Oliveira, P.A.; Pires, M.J.; Neuparth, M.J.; Lanzarin, G.; Félix, L.; Venâncio, C.; de Pinto, M.L.; Ferreira, J.; Gaivão, I.; et al. Effect of a Sub-Chronic Oral Exposure of Broccoli (Brassica Oleracea L. Var. Italica) By-Products Flour on the Physiological Parameters of FVB/N Mice: A Pilot Study. Foods 2022, 11, 120. [Google Scholar] [CrossRef]
- Saavedra-Leos, M.Z.; Leyva-Porras, C.; Toxqui-Terán, A.; Espinosa-Solis, V. Physicochemical Properties and Antioxidant Activity of Spray-Dry Broccoli (Brassica Oleracea Var Italica) Stalk and Floret Juice Powders. Molecules 2021, 26, 1973. [Google Scholar] [CrossRef] [PubMed]
- Salas-Millán, J.-Á.; Aznar, A.; Conesa, E.; Conesa-Bueno, A.; Aguayo, E. Functional Food Obtained from Fermentation of Broccoli By-Products (Stalk): Metagenomics Profile and Glucosinolate and Phenolic Compounds Characterization by LC-ESI-QqQ-MS/MS. LWT 2022, 169, 113915. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Bansal, S.; Dhillon, G.S. Enhanced β-Galactosidase Production by Supplementing Whey with Cauliflower Waste. Int. J. Food Sci. Technol. 2008, 43, 1499–1504. [Google Scholar] [CrossRef]
- Monllor, P.; Muelas, R.; Roca, A.; Atzori, A.S.; Díaz, J.R.; Sendra, E.; Romero, G. Long-term Feeding of Dairy Goats with Broccoli By-Product and Artichoke Silages: Milk Yield, Quality and Composition. Animals 2020, 10, 1670. [Google Scholar] [CrossRef]
- Muelas, R.; Romero, G.; Díaz, J.R.; Monllor, P.; Fernández-López, J.; Viuda-Martos, M.; Cano-Lamadrid, M.; Sendra, E. Quality and Functional Parameters of Fermented Milk Obtained from Goat Milk Fed with Broccoli and Artichoke Plant By-Products. Foods 2022, 11, 2601. [Google Scholar] [CrossRef]
- Mueller, K.; Blum, N.M.; Kluge, H.; Mueller, A.S. Influence of Broccoli Extract and Various Essential Oils on Performance and Expression of Xenobiotic- and Antioxidant Enzymes in Broiler Chickens. Br. J. Nutr. 2012, 108, 588–602. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.H.; Wang, D.G.; Pan, H.Y.; Zheng, W.B.; Zuo, A.Y.; Liu, J.X. Effects of Broccoli Stem and Leaf Meal on Broiler Performance, Skin Pigmentation, Antioxidant Function, and Meat Quality. Poult. Sci. 2012, 91, 2229–2234. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Ozdal, T.; Tomas, M.; Capanoglu, E. Oil Matrix Modulates the Bioaccessibility of Polyphenols: A Study of Salad Dressing Formulation with Industrial Broccoli by-Products and Lemon Juice. J. Sci. Food Agric. 2022, 102, 5368–5377. [Google Scholar] [CrossRef]
- Drabińska, N. The Evaluation of Amino Acid Profiles in Gluten-Free Mini Sponge Cakes Fortified with Broccoli By-Product. Separations 2022, 9, 81. [Google Scholar] [CrossRef]
- Drabińska, N.; Nogueira, M.; Szmatowicz, B. Valorisation of Broccoli By-Products: Technological, Sensory and Flavour Properties of Durum Pasta Fortified with Broccoli Leaf Powder. Molecules 2022, 27, 4672. [Google Scholar] [CrossRef]
- Ying, D.; Sanguansri, L.; Cheng, L.; Augustin, M.A. Nutrient-dense Shelf-stable Vegetable Powders and Extruded Snacks Made from Carrots and Broccoli. Foods 2021, 10, 2298. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabińska, N.; Baczek, N.; Šimková, K.; Starowicz, M.; Jeliński, T. Application of Broccoli Leaf Powder in Gluten-Free Bread: An Innovative Approach to Improve Its Bioactive Potential and Technological Quality. Foods 2021, 10, 819. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabińska, N.; Rosell, C.M.; Fadda, C.; Anders, A.; Jeliński, T.; Ostaszyk, A. Broccoli Leaf Powder as an Attractive By-Product Ingredient: Effect on Batter Behaviour, Technological Properties and Sensory Quality of Gluten-Free Mini Sponge Cake. Int. J. Food Sci. Technol. 2019, 54, 1121–1129. [Google Scholar] [CrossRef] [Green Version]
- Drabińska, N.; Ciska, E.; Szmatowicz, B.; Krupa-Kozak, U. Broccoli By-Products Improve the Nutraceutical Potential of Gluten-Free Mini Sponge Cakes. Food Chem. 2018, 267, 170–177. [Google Scholar] [CrossRef]
- Costa, C.; Lucera, A.; Marinelli, V.; del Nobile, M.A.; Conte, A. Influence of Different By-Products Addition on Sensory and Physicochemical Aspects of Primosale Cheese. J. Food Sci. Technol. 2018, 55, 4174–4183. [Google Scholar] [CrossRef]
- Lucera, A.; Costa, C.; Marinelli, V.; Saccotelli, M.A.; del Nobile, M.A.; Conte, A. Fruit and Vegetable By-Products to Fortify Spreadable Cheese. Antioxidants 2018, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Bravo, P.; Abellán, Á.; Zapata, P.J.; García-Viguera, C.; Domínguez-Perles, R.; Giménez, M.J. Broccoli Products Supplemented Beers Provide a Sustainable Source of Dietary Sulforaphane. Food Biosci. 2023, 51, 102259. [Google Scholar] [CrossRef]
- Prokopov, T.; Goranova, Z.; Baeva, M.; Slavov, A.; Galanakis, C.M. Effects of Powder from White Cabbage Outer Leaves on Sponge Cake Quality. Int. Agrophys. 2015, 29, 493–500. [Google Scholar] [CrossRef]
- Stojceska, V.; Ainsworth, P.; Plunkett, A.; Ibanoǧlu, E.; Ibanoǧlu, S. Cauliflower By-Products as a New Source of Dietary Fibre, Antioxidants and Proteins in Cereal Based Ready-to-Eat Expanded Snacks. J. Food Eng. 2008, 87, 554–563. [Google Scholar] [CrossRef]
- Femenia, A.; Robertson, J.A.; Waldron, K.W.; Selvendran, R.R. Cauliflower (Brassica Oleracea L), Globe Artichoke (Cynara Scolymus) and Chicory Witloof (Cichorium Intybus) Processing by-Products as Sources of Dietary Fibre. J. Sci. Food Agric. 1998, 77, 511–518. [Google Scholar] [CrossRef]
- Llorach, R.; Tomás-Barberán, F.A.; Ferreres, F. Functionalisation of Commercial Chicken Soup with Enriched Polyphenol Extract from Vegetable By-Products. Eur. Food Res. Technol. 2005, 220, 31–36. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, Y.; Li, X.; Kang, H. Antioxidant Extract from Cauliflower Leaves Effectively Improve the Stability of Pork Patties during Refrigerated Storage. J. Food Process. Preserv. 2020, 44, e14510. [Google Scholar] [CrossRef]
- Vázquez-Durán, A.; Gallegos-Soto, A.; Bernal-Barragán, H.; López-Pérez, M.; Méndez-Albores, A. Physicochemical, Nutritional and Sensory Properties of Deep Fat-Fried Fortified Tortilla Chips with Broccoli (Brassica Oleracea L. Convar. Italica Plenck) Flour. J. Food Nutr. Res. 2014, 53, 313–323. [Google Scholar]
- González-Ballesteros, N.; Vidal-González, J.; Rodríguez-Argüelles, M.C. Wealth from By-Products: An Attempt to Synthesize Valuable Gold Nanoparticles from Brassica Oleracea Var. Acephala Cv. Galega Stems. J. Nanostruct. Chem. 2021, 11, 635–644. [Google Scholar] [CrossRef]

| By-Product Characteristics | F (kHz) | Power Parameters | Solvent | S:L Ratio (w:v) | T (min) | T (°C) | Other Information | Main Findings | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Radish seeds cv. IPR 11 Particle size information NA | 25 | 165 W | EtOH | 1:12 | 20–60 | 30–60 | USAE bath with indirect contact. After the extraction, seeds were separated by filtration, and the excess solvent was removed until reaching a constant weight. | The maximum yield (25%), a greater amount of phytosterols and tocopherols, and, consequently, greater oxidative stability. | [54] |
| Red radish cv. information NA Freeze dried 1–2 mm pieces | NA | 138–358 W | H2O | 0.06:30 | 30–120 | 45 | Before USAE by pulse cycles of 5 s on and 1 s off, extraction of anthocyanins was performed. | High-energy USAE treatment (120 min at 286–258 W) is adequate to enhance TAC but does not preserve anthocyanins. | [49] |
| Broccoli leaves, stems, and inflorescences cvs.: ‘TSX 007′, ‘Monaco’, ‘BRO 2047′, ‘Parthenon’, and ‘Summer Purple’ Dried (45 °C, 48 h) Particle size information NA | NA | NA | 80% EtOH | 10:60 | 60 | 45–50 | Excess EtOH was removed by heating it at 37 °C in a rotary evaporator under vacuum. The resulting aqueous extracts were combined and lyophilized. | Extraction yield of 13.4–16.3% dw. High TAC and chlorophylls and phenolics (mainly kaempferol and quercetin glucosides) in leaf extracts (‘Summer Purple’) and high GLS content in inflorescence extract. | [24] |
| Broccoli leaves, stems, and inflorescences cv. Parthenon Dried (45 °C, 24–48 h) Particle size information NA | NA | 220 V 360 W | H2O | 1:50 | 60 | NA | Before USAE, the mixture was heated for 16 min at 121 °C. After US, four times its volume of ethanol was added, and after 12 h of incubation, it was dried at 45 °C in a forced-air oven. | USAE did not manage to modify the neutral sugar profile. | [55] |
| Broccoli by-products cv. information NA Dried (35 °C, 48 h) Particle size information NA | 25 | 50 W/L | H2O | 1:10 | 60 | 15 | The extract was dried at 30 °C in a vacuum oven. The residue was mixed with water and recovered by centrifugation (6000 rpm × 10 min). | USAE extracted more bioactive compounds than supercritical fluids but not as many as pressurized liquid. | [53] |
| Cauliflower by-products cv., drying, and particle size information NA | NA | 175 W | H2O (pH 11) | 1:4 | 15 | NA | The crude fiber and insoluble protein were removed from the extract first with 3 layer gauze and then by centrifugation (4000 rpm × 30 min). | Extraction yield of 53.1% and 12.066 g of soluble leaf protein kg−1. | [23] |
| Cauliflower by-products Blanching cv. information NA Dried (50–55 °C overnight) Particle size 0.5 mm | 24 | 400 W | H2O 70% MeOH 80% Ac | 50:100 | 0–10 | NA | Amplitude USAE from 20–100%. After US, centrifugation at 1500× g for 15 min, and the pellet was centrifuged with 100 mL of solvent. Both supernatants were collected, combined, and filtered under vacuum conditions. | The amplitude affected the extraction of isothyocyanates (80% amplitude for 3 min) and phenolics (100% amplitude for 3 min). | [52] |
| Rapeseed meal cv., drying, and particle size information NA | 28 | 0.228 W/cm2 | H2O | 1:30 | 41.48 | NA | Other extraction conditions were pH 11.71 and USAE power 40%. | High protein yield of 43.3% and nitrogen solubility of 18.1%. | [44] |
| Broccoli cv., drying, and particle size information NA | 40 | 500 W | Ch 80% EtOH Ac | 100:500 | 60 | 40 | Extracts were combined to metal-organic framework nanocubes. They were dispersed by an ultrasonic probe in 100 mL, then triethylamine as a capping agent was added, and the mixture was agitated and heated for 12 h at 130 °C. | Broccoli extract combined with MOF-5-NCs showed synergistic activity against P. aeruginosa bacteria in standard and clinical strains. | [43] |
| Kale cv. information NA Convective dryer (39 °C) Particle size information NA | 20 | 100 W | 80% EtOH | 2:40 | 60 | 60 | USAE in two cycles of 30 min Extracts were filtered, combined, and evaporated. The residues were dissolved in methanol and filtered. | High isolation of phenolic acids and high yield of biocompounds in short time and reduced solvent volume with easy handling. | [45] |
| Broccoli seeds cv., drying, and particle size information NA | NA | 200–500 W | H2O EA | 1:10–1:50 | 5–40 s | 25–35 | Before USAE, broccoli seeds were treated in a MWAE oven for 1–4 min at low power. | The highest SFN formation was under a MWAE pretreatment of 3 min and a US treatment of 20 s, 500 W, and 1:10 for water or 1:50 ethyl acetate. | [48] |
| Broccoli stems and leaves cv. information NA. Dried (30–35 °C, 48 h). Particle size information NA | 25 | 50 W/L | H2O | 1:10 | 60 | NA | After homogenization, the extract was dried at 30 °C in a vacuum oven. The residue was mixed with water (25 mL) and recovered by centrifuging at 6000 rpm for 10 min. | High-quality extract in terms of antimicrobial efficacy against Pseudomonas spp. and Candida krusei. | [56] |
| White cabbage cv. information NA Oven-dried (60 °C, 72 h) Particle size information NA | 40 | 132 W | 60% EtOH | 2:10 | 120 | 30–70 | Ultrasonic intensity of 0.46 W/cm2. The obtained extracts were hydrolyzed before analyzing. | Richer extract at 30 °C. Antimicrobial activities only of the hydrolyzed extracts. | [46] |
| Broccoli heads cv., drying, and particle size information NA | 23 | NA | H2O | 1:20 | 1–12 | 25–60 | Amplitude was set at 135 µm. | Higher myrosinase inactivation and SFN content at 60 °C for 4 min. Activation energy was 3.6-fold lower regarding traditional blanching. | [50] |
| Camelina sativa oil cv., drying, and particle size information NA | 35 | 60–120 W | 40–80 EtOH | 1:5–1:15 | 10–20 | 30 | USAE in 2–4 cycles of 5 min each. A solid-phase extraction procedure to obtain an extract rich in GLS and to perform cellular assays. | High-GLS extraction with 65% EtOH, 1:15, and 10 min. The purified extract (800 mg from 10 g) showed chemopreventive action against colorectal cancer cells. | [47] |
| Thirty-six Brassica oleracea var. acephala accessions Dried in an oven (105 °C) or freeze-dried Particle size information NA. | 40 | 300 W | 80% MetOH | 0.03:1.5 | 30 | 20 | After USAE, extracts were centrifuged at 15,000× g for 5 min. | Higher GLS content, TAC, TPC, and sugars with freeze-dried samples and USAE compared with hot extraction. | [51] |
| Cabbage leaves, fresh and steamed (100 °C, 2 min) cv., and drying info NA Particle size 1.7–2.55 mm. | 37 | 320 W | H2O | 5:50 | 40 | NA | Absorbed US power of 0.03 W/g extraction + MWAE or vaccum. | Higher glucoraphanin content with USAE + vacuum or MWAE More effective (87%) when leaves were steamed, presenting higher myrosinase inactivation. | [18] |
| By-Product Characteristics | Power (W) | P | Solvent | S:L Ratio (w:v) | T (min) | T (°C) | Other Information | Main Findings | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Purple-heart radish cv. information NA. Dried in the oven (60 °C) Particle size 117-μm. | NA | Atm | H2O andEtOH | 0.5:31.5 | 20 | 70 | Twenty grams of broccoli powder were pre-extracted with petroleum ether II at 80 °C for 6 h. | Polysaccharide yield (29%) was higher than hot (~24%) and USAE (27%) extraction. | [58] |
| White cabbage leaves are chopped. cv. information NA. Fresh or dried with a hot air dryer (60 °C) Particle size information NA. | 130–390 | Atm | DCh H2O | 5:50 | 1–5 | 22–38(DCh)22–98(H2O) | After extraction with a domestic MW oven, the extract was filtered and dehydrated using the rotary evaporator at 30 °C (for DCh) or 45 °C (for H2O). | Higher SFN yield in less time. Higher MW powers resulted in a shorter extraction time.No differences between fresh and semi-dried samples, nor between the solvents used. | [61] |
| Broccoli florets, stems, and leaves. cv., drying, and particle size information NA. | NA | Atm | 40–80% MetOH | 1:20 | 10–20 | 55–75 | After extraction, the mixture was centrifuged for 20 min at 10,350 rpm and 4 °C. The supernatant was filtered and stored at −20 °C. | The optimum conditions were 74.5, 80, 80% MetOH, 15.9, 10, 18.9 min, and 74.5, 73.3, 75 °C for stalks, leaves, and florets, respectively. Increased the phenolic yield up to 65.3, 45.70, 133.6% for stalks, leaves, and florets, respectively, in less time. | [62] |
| Purple and white cabbages cv. information NA. Sun-dried. Particle size 80–100 µm. | 200–400 | Atm | NAc | 1:4–1:7 | 10–25 | 60–90 | After extraction, the extract was completed with 10 mL. | Optimum conditions: 201 W at 60 °C for 10 min at a 1:4 ratio. A polynomial regression was the best-fitting model. | [57] |
| Cabbage leaves (1.7–2.55 mm) cv. information NA. Fresh and steamed. (100 °C for 2 min). Particle size information NA. | 180 | Atm 70 kPa | H2O | 5:50 | 10 | NA | Combined with USAE | Higher glucoraphanin content using vacuum MWAE with USAE than atmospheric MWAE. More effective (87%) when leaves were previously steamed, and a higher inactivation of the myrosinase enzyme. | [18] |
| By-Product Characteristics | Combined with | Enzymes | Inactivation Enzymes | S:L Ratio (w:v) | T (min) | T (°C) | Main Findings | Ref. |
|---|---|---|---|---|---|---|---|---|
| Purple-heart radish cv. information NA. Oven-dryer (60 °C). | MW | Papain | NA | 1:55–1:65 | 8.4 | 68 | EAE combined with MWAE facilitated cell rupture and enzymolysis, improving the extraction yields and shortening the extraction time. | [58] |
| Broccoli by-products (leaves, stems, and inflorescences). cv. Parthenon Forced-air oven dryer (45 °C, 24–48 h) | NA | Cellulase | Cooled at room temperature | 1:50.8 | 120 | 50 | Decreased the sugar content and increased the uronic acid content. Non-extractable phenolics were found higher in inflorescences and increased with EAE and TAC. | [55] |
| Radish root ground with a mortar. cv. and drying information NA. | US | Cellulases Pectinases Amylases Glucanases Hemicellulases | Few minutes at 90 °C | 10:40 | 66–84 | 46–64 | Higher TAC with the highest extraction of TPC. | [60] |
| Canola (Brassica napus) oil pressing residues. Particle size: 0.5 mm cv. and drying information NA. | NA | Protamex® Alcalase® Viscozyme® Phyzyme® | NA | 1:10 | 240–1200 | 45–50 | The applied enzymes effectively enhanced the solubility of proteins, despite the lower yield of crude proteins compared to the alkaline extraction (40–82 vs. 91 g/100 g dw). | [63] |
| Cauliflower florets and leaves cv. information NA Pre-extraction with 96% ethanol (1:5) for 30 min at 100 °C. Residue was dried at 40 °C. | NA | Proteases Cellulases Endopolygalacturonase II Rhamnogalacturonan hydrolase Pectin methyl esterases Rapidase Liq+ | 10 min at 100 °C | 5:500 | 240 | 50 | Higher methoxy pectins of high molar mass were extracted with three enzyme mixtures. Health benefit pectic oligosaccharides were obtained after pectin extraction. Seventy percent of the by-products were consumed to extract two products of interest. | [64] |
| Cabbage (91.5% humidity) | NA | Blakeslea trispora (mould) | NA | 1:10 | NA | 26 | Higher biomass accumulation and carotenoid production. | [59] |
| By-Product Characteristics | Green Technology Used | S:L Ratio (w:v) | T (min) | T (°C) | Other Parameters to Be Monitored | Main Findings | Ref. |
|---|---|---|---|---|---|---|---|
| Broccoli leaves, stems, and inflorescences. cv. ParthenonDried in a forced-air oven (45 °C, 24–48 h). | Supercritical fluids using CO2 | NA | 120 | 45–55 | Dynamic extraction. Flow: 2 L/min. Three hundred bar at 55 °C or one-hundred and fifty bar at 45 °C. | The content of non-extractable phenolics and TAC increased and were higher in inflorescences. | [55] |
| Broccoli by-products. Dried (35 °C, 48 h). | Supercritical fluids using CO2 | NA | 140 | 35 | Two pumps:
Flow: 2 L/min | Presented the worst results regarding the extraction of bioactive compounds. | [67] |
| Broccoli by-products. Dried (35 °C, 48 h) | Pressurized liquid | 15:25 | 10 | 60 | Steps:
| The highest content of bioactive compounds and TAC. | [53] |
| Yellow mustard flour (30.7% oil, 30.9% protein, 4% ash, and 9% fiber). | Ultrafiltration | NA | NA | 25 | Before ultrafiltration, defatting was carried out with hexane. Film composite membrane (150–300 Da, pH tolerance range 2–10 at 25 °C, max. Tª of 80 °C, and pressure of 40 bar). | In acidic conditions, 77% of the phenolic compounds were recovered. Combination of diafiltration with nanofiltration was beneficial only when processing under acidic conditions. | [68] |
| Broccoli stems and leaves Dried (30–35 ℃, 48 h). | Supercritical fluids using CO2 | NA | 140 | 35 | Two pumps:
Flow: 2 L/min Drying in a vacuum oven (30 °C) | High-quality extract in terms of antimicrobial efficiency against Pseudomonas spp. and Candida krusei. | [56] |
| Broccoli stems and leaves cv. Parthenon and Naxos. | Supercritical fluids using CO2 | NA | NA | NA | Two pumps:
| High yield of β-carotene, phenolic compounds, chlorophylls, and phytosterols. Great TAC. Reduced organic solvent consumption. | [69] |
| Brassica Species | Matrix | By-Product Characteristics | Formulation Incorporation | Benefits | Ref. |
|---|---|---|---|---|---|
| Broccoli (Brassica oleracea var. italica) | Salad dressing recipes | Stems and leaves cv. Marathon No pre-blanching Freeze-dried Grounded fine powder | Powder:lemon juice:oil (olive, hazelnut, or sunflower) ratio (1:2.5:7.5; w:v:v). | Higher bioaccessibility of polyphenols from broccoli in an oil matrix. | [84] |
| Durum Pasta | Leaves cv. Sebastian Blanching Freeze-drying Particle size ≤0.60 mm | Durum semolina flour, water, olive oil, and salt. Leaves powder: 0–5%. | Decreased cooking time and water absorption. Increased the swelling index. Firmness and total shearing force decreased. Greener than control. Higher dimethyl sulphide and mineral content. No effect on overall acceptance. | [86] | |
| Gluten-Free Sponge Cakes | Mature leaves cv. Sebastian Blanching in hot water Freeze-dried Particle size ≤0.60 mm | Potato and corn starch, eggs, sugar, oil, salt, and baking powder. Leaves powder: 0–7%. | Good source of free amino acids. Promising product for a gluten-free diet. | [85] | |
| Powders and extruded snacks | Broccoli pomace Steam blanching Freeze-drying Particle size: 800 µm sieve | Dried and wet pomace are used for extrusion. Vegetable powder:rice flour ratio (100:0, 80:20, 60:40, 40:60, 20:80, and 0:100). Maximum wet pomace: 3%. | Enhancement of the nutritional properties. Powders were richer in fiber but contained less total carbohydrates. A reduced expansion of extruded snacks with increasing vegetable levels in the formulation. | [87] | |
| Gluten-free bread | Mature leaves cv. Sebastian Blanching in hot water Freeze-dried Particle size ≤0.60 mm | Corn starch, potato starch, sugar, fresh yeast, pectin, rapeseed oil, salt, and water. By-products (5%) instead of corn starch. | Higher content of proteins and minerals. Improved specific volume and bake loss. Improved TAC and anti-aging activity. | [88] | |
| Gluten-free mini sponge cake | Mature leaves cv. Sebastian Blanching in hot water Freeze-dried Particle size ≤0.60 mm | Consists of 30.6% potato and 7.8% corn starches, 43% egg, 14% sugar, 3.7% sunflower oil, 0.2% salt, and 0.7% gluten-free baking powder. The inclusion was: 2.5–7.5% (w/w) | Increase of firmness. No changes in sensorial quality. Sample with 2.5% was distinguished. | [89] | |
| Gluten-free mini sponge cake | Mature leaves cv. Sebastian Blanching in hot water Freeze-dried Particle size ≤0.60 mm | Consists of 30.6% potato and 7.8% corn starches, 43% egg, 14% sugar, 3.7% sunflower oil, 0.2% salt, and 0.7% gluten-free baking powder. The inclusion was: 2.5–7.5% (w/w). | Increased GLS content and TAC. Optimal improvement with addition of 2.5% as starch substitute. | [90] | |
| Deep fat-fried fortifiedtortilla chips | Waste cv. Plenck Dehydrated wastes Particle size <250 µm | Broccoli flour: 2–8%. | Increased contents of protein (from 8.1 to 9.5%), crude fiber (from 1.9 to 3.1%), lysine (from 25.6 to 35.1 g kg−1), and calcium (from 0.45 to 0.73 g kg−1). A 10.5% lower final oil content. | [99] | |
| Primosale cheese | Dried (30 °C, 48 h) Fine powder | 50 and 100 g kg−1 | Better nutritional properties, friability, and adhesiveness. | [91] | |
| Spreadable cheese | Stalks and leaves Dried (30 °C, 48 h) Fine powder | 50 and 100 g kg−1 | Increased TPC, TFC, and TAC. | [92] | |
| Fish burgers | Dried (35 °C, 48 h) Hammer mill | Extracted by USAE. Spray-dried: maltodextrins, wall material (10–30%), the core/wall material ratio (1:2, 1:5, 1:10, 1:20), and T: 80–170 °C. Minced fish is mixed with 5% w/w of microencapsulated extract. | Increased TPC and TAC, even if cooked. | [53] | |
| Beer | Powder | Supplementation of 0.1% powder (w/v). After 3 days at 10 °C, the by-product was removed and the beers remained in fermentation until day 14, which then ended. | Higher SFN content (2.54 mg/L, prior to bottling). SFN remained stable until bottling, when concentrations decreased by >50%. After 150 days, the SFN content was 0.30 mg/L in beers supplemented with powder. | [93] | |
| Kale (Brassica oleracea var. sabellica) + Broccoli (Brassica oleracea var. italica) | Kale pesto sauce | Leaves Vaccum-packaged Blanching in a water bath | Kale pesto with kale leaves. Kale pesto with kale and bimi broccoli by-products. Mustard was included. | No influence on sensory quality. Glucoraphanin content was enhanced. Including mustard showed better microbial quality and color preservation after 20 days at 5 °C, without sensory alterations. | [12] |
| Cabbage (Brassica oleracea var. capitata) | Gold nanoparticles | Stems cv. Galega Shade-drying at room temperature | Aqueous extraction (1:2 w:v), (100 °C, 15 min) + frozen. Different volumes of an aqueous solution of HAuCl4 (0.01 M) were added to a fixed volume of extract. | Higher TPC and TAC. | [100] |
| Sponge cake | Leaves White cabbage Blanching in hot water: Cabbage:water 1 g per 7 cm3 Dried in the oven (80 °C, 6 h) Particle size <200 µm | Eggs, sugar, and wheat flour.A double mixing procedure: dividing the whipping of egg whites and egg yolks. A by-product was added to substitute between 10 and 20% of the wheat flour. | Lower springiness of cakes and crumb tenderness. The structure was stable at high loads (lower shrinkage). Nutritional value decreased. | [94] | |
| Cauliflower (Brassica oleracea var. botrytis) | Ready-to-eat snack | Florets, curd, stem, and leaves Oven-dried (80 °C, 10 h) Particle size: 0.5 mm mesh | Consists of 35.6% wheat flour, 20% corn starch, 10% oat flour, 10% egg whites, 10% milk powder, 3% onion powder, 5% tomato powder, 5% carrot powder, 0.1% dill, 0.1% mint, and 0.4% salt. Wheat flour was replaced with dried cauliflower: 5–20%. | Levels of 5–20% increased dietary fiber, protein content, and water absorption index. Significant effects on the expansion indices, bulk density, color, and total cell area. The taste panel acceptability score showed that cauliflower by-products could be added up to 10%. | [95] |
| Commercial chicken soup | Leaves and stems | Extraction by boiling water (1:4 w/v) (1 h) and freeze-dried. Addition: 2.5–10 mg extract/mL soup. | The best concentration was 5 mg of extract/mL of soup. TAC increased between 3.5- and 13-fold (ABTS·+ assay) as well as between 23- and 85-fold (FRAP assay). | [97] | |
| Carrot paté | Floret/curd and stem Convection oven (between 40 and 75 °C) Particle size <100 µm | Consists of 62.5% pulverized carrot, 15% whole egg, 3.4% margarine, 9% water, 1.2% lemon juice, 1.7% sugar, 3% milk powder, 2.7% starch, 0.8% carrageenan, 1.4% salt, 0.03% riboside, and 0.08% pepper. Carrot paté (1.8%) and carob-carrageenan (0.2%) were replaced by 2% (w/w) by-products. | Products underwent discoloration (more yellowish) and a decrease in firmness and adherence, which could limit their potential as fiber supplements. Hardness and adherence decreased in floret and stem formulations. | [96] | |
| Quiche ‘Lorraine’ | Floret/curd and stem Convection oven (between 40 and 75 °C) Particle size <100 µm | Consists of 29.3% water, 22% whole eggs, 20% cream, 8% ham, 8% onions, 6% cheese, 3% milk powder, 2% starch, 1% oil, 0.6% salt, and 0.1% pepper. Consists of 2% cauliflower fiber instead of dried whole egg (1.5%) and starch (0.7%). | The quiche containing florets and stems had a cauliflower flavor, although the overall texture was less gelled, especially for the stem samples, but the color was not affected. The quiche was considered suitable for addition of fibers. | [96] | |
| Meat products | Floret/curd and stem Convection oven (between 40 and 75 °C) Particle size <100 µm | Beefburgers were prepared by adding 2% (w/w) of a fiber preparation. | Improvement of the yield (10%) for stalk and floret samples. Firmness was improved when stem and floret were added. | [96] | |
| Bechamel sauce | Floret/curd and stem Convection oven (between 40 and 75 °C) Particle size <100 µm | Consists of 71.1% water, 8% milk powder, 4% margarine, 3.1% flour, 1.5% starch, 1% egg yolk, 10.3% fat, 0.77% salt, and 0.09% pepper. Inclusion: 3% (before or after cooking). | Viscosity increased when cauliflower fiber was added before cooking (in the case of the floret and mainly the stem). The effectiveness of supplementation depends on the time of their incorporation (before or after cooking). Modifications to color, texture, and cauliflower flavor in sensorial analysis. | [96] | |
| Tomato sauce | Floret/curd and stem Convection oven (between 40 and 75 °C) Particle size <100 µm | Consists of 69.4% water, 18% tomato concentrate, 6% carrot puree, 1% onion powder, 1% flour, 0.5% starch, 0.07% garlic powder, 0.75% salt, 0.05% pepper, 1% sugar, 2% olive oil, and 0.25% xanthan. Inclusion: 2% (w/w) of fiber-enriched materials and 0.15% xanthan. | It was designed to test whether cauliflower could partially substitute for xanthan as a thickening agent. The samples presented a granular texture, which limited their use except for their incorporation in ‘bolognese’ type sauces. | [96] | |
| Pork patties | Leaves Dried in a vacuum oven (45 °C, 8 h) | Consists of 50 g of califlower leaves ground + 500 mL (80% EtOH). Incorporation: 2.5–10 g/kg by-product extracts, or 0.2 g/kg BHA. | Higher TPC and DPPH values and lower TBARS values and protein carbonyl contents. Microbial growth was retarded. | [98] | |
| Apple juice (total sugar of 9.2 g/100 mL) | Steam and leaves Blanching with hot water Dried (10 min, 50–55 °C) Particle size: 0.5 mm mesh | Extracted by USAE (Table 1). Cauliflower extracts: 0–40%. | They are appropriate, containing up to 10% extract. Nutritional value was improved by enhancing isothiocyanates. Differences in smell and taste with 20% and 40% extracts. | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artés-Hernández, F.; Martínez-Zamora, L.; Cano-Lamadrid, M.; Hashemi, S.; Castillejo, N. Genus Brassica By-Products Revalorization with Green Technologies to Fortify Innovative Foods: A Scoping Review. Foods 2023, 12, 561. https://doi.org/10.3390/foods12030561
Artés-Hernández F, Martínez-Zamora L, Cano-Lamadrid M, Hashemi S, Castillejo N. Genus Brassica By-Products Revalorization with Green Technologies to Fortify Innovative Foods: A Scoping Review. Foods. 2023; 12(3):561. https://doi.org/10.3390/foods12030561
Chicago/Turabian StyleArtés-Hernández, Francisco, Lorena Martínez-Zamora, Marina Cano-Lamadrid, Seyedehzeinab Hashemi, and Noelia Castillejo. 2023. "Genus Brassica By-Products Revalorization with Green Technologies to Fortify Innovative Foods: A Scoping Review" Foods 12, no. 3: 561. https://doi.org/10.3390/foods12030561