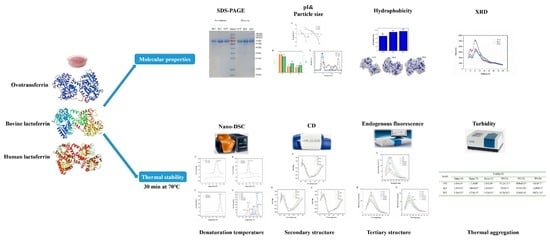
Providing New Insights on the Molecular Properties and Thermal Stability of Ovotransferrin and Lactoferrin
Abstract
:1. Introduction
2. Material and Method
2.1. Materials and Reagents
2.2. Preparation of Ovotransferrin
2.3. Molecular Weight Distribution
2.4. Determination of Sulfhydryl Content
2.5. Determination of Particle Size and Potential
2.6. Measurements of Surface Hydrophobicity
2.7. X-ray Diffraction (XRD)
2.8. Nano Differential Scanning Calorimetry (Nano DSC)
2.9. Circular Dichroism
2.10. Endogenous Fluorescence Spectroscopy
2.11. Determination of Turbidity
2.12. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Molecular Properties
3.1.1. Molecular Weight Distribution
3.1.2. Determination of Sulfhydryl Content
3.1.3. Determination of Particle Size and Potential
3.1.4. Surface Hydrophobicity (H0)
3.1.5. X-ray Diffraction Characterization
3.2. Thermal Stability Analysis
3.2.1. Nano Differential Scanning Calorimetry (DSC) Characterization
3.2.2. Circular Dichroism (CD) Analysis
3.2.3. Endogenous Fluorescence Spectroscopy Analysis
3.2.4. Determination of Turbidity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sreedhara, A.; Flengsrud, R.; Prakash, V.; Krowarsch, D.; Langsrud, T.; Kaul, P.; Devold, T.G.; Vegarud, G.E. A comparison of effects of pH on the thermal stability and conformation of caprine and bovine lactoferrin. Int. Dairy J. 2010, 20, 487–494. [Google Scholar] [CrossRef]
- Talukder, J. Egg Derived Ovotransferrins and Lactoferrins. In Nutraceuticals in Veterinary Medicine; Gupta, R.C., Srivastava, A., Lall, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 235–243. [Google Scholar] [CrossRef]
- Narmuratova, Z.; Hentati, F.; Girardet, J.M.; Narmuratova, M.; Céline, C.K. Equine lactoferrin: Antioxidant properties related to divalent metal chelation. LWT 2022, 161, 113426. [Google Scholar] [CrossRef]
- Tsuda, H.; Kozu, T.; Iinuma, G.; Ohashi, Y.; Saito, Y.; Saito, D.; Iigo, M. Cancer prevention by bovine lactoferrin: From animal studies to human trial. Biometals 2010, 23, 399–409. [Google Scholar] [CrossRef]
- Chen, R.; Huang, L.; Zheng, W.; Zhang, M.; Xin, Z.; Liu, L.; Chen, Z. Lactoferrin ameliorates myocardial fibrosis by inhibiting inflammatory response via the AMPK/NF-κB pathway in aged mice. J. Funct. Foods 2022, 93, 105106. [Google Scholar] [CrossRef]
- Comstock, S.S.; Reznikov, E.A.; Contractor, N.; Donovan, S.M. Dietary bovine lactoferrin alters mucosal and systemic immune cell responses in neonatal piglets. J. Nutr. 2014, 144, 525–532. [Google Scholar] [CrossRef] [Green Version]
- Vongbhavit, K.; Underwood, M.A. Prevention of necrotizing enterocolitis through manipulation of the intestinal microbiota of the premature infant. Clin. Therapeut. 2016, 38, 716–732. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Wang, J.; Bu, D.; Liu, G.; Zhang, C.; Wei, H.; Zhou, L.; Wang, J. Factors Affecting the Lactoferrin Concentration in Bovine Milk. J. Dairy Sci. 2008, 91, 970–976. [Google Scholar] [CrossRef] [Green Version]
- Oberčkal, J.; Liaqat, H.; Matijašić, B.; Rozman, V.; Treven, P. Quantification of lactoferrin in human milk using monolithic cation exchange HPLC. J. Chromatogr. B 2023, 1214, 123548. [Google Scholar] [CrossRef]
- Soyeurt, H.; Bastin, C.; Colinet, F.; Arnould, V.; Berry, D.; Wall, E.; Dehareng, F.; Nguyen, H.; Dardenne, P.; Schefers, J.; et al. Mid-infrared prediction of lactoferrin content in bovine milk: Potential indicator of mastitis. Animal 2012, 6, 1830–1838. [Google Scholar] [CrossRef] [Green Version]
- Blanca, F.; Edward, A.; Tania, S.; Quintín, R. Lactoferrin as a nutraceutical protein from milk, an overview. Int. Dairy J. 2019, 89, 37–41. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, K. Analysis on the biological characteristics and industrialization technology of lactoferrin. China Dairy 2020, 10, 58–59. [Google Scholar] [CrossRef]
- Wu, J.; Acero-Lopez, A. Ovotransferrin: Structure, bioactivities, and preparation. Food Res. Int. 2012, 46, 480–487. [Google Scholar] [CrossRef]
- Zhang, X.; Yue, X.; Ma, B.; Fu, X.; Ren, H.; Ma, M. Ultrasonic pretreatment enhanced the glycation of ovotransferrin and improved its antibacterial activity. Food Chem. 2021, 346, 128905. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Rupa, P.; Kovacs Nolan, J.; Turner, P.V.; Matsui, T.; Mine, Y. Oral Administration of Hen Egg White Ovotransferrin Attenuates the Development of Colitis Induced by Dextran Sodium Sulfate in Mice. J. Agric. Food Chem. 2015, 63, 1532–1539. [Google Scholar] [CrossRef]
- Lee, J.; Ahn, D.U.; Paik, H.D. In Vitro Immune-Enhancing Activity of Ovotransferrin from Egg White via MAPK Signaling Pathways in RAW 264.7 Macrophages. Korean J. Food Sci. Anim. Resour. 2018, 38, 1226–1236. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Moon, S.H.; Kim, H.S.; Park, E.; Ahn, D.U.; Paik, H.D. Antioxidant and anticancer effects of functional peptides from ovotransferrin hydrolysates. J. Sci. Food Agric. 2017, 97, 4857–4864. [Google Scholar] [CrossRef] [PubMed]
- Shang, N.; Wu, J. Egg White Ovotransferrin Shows Osteogenic Activity in Osteoblast Cells. J. Agric. Food Chem. 2018, 66, 2775–2782. [Google Scholar] [CrossRef] [PubMed]
- Henchion, M.; Moloney, A.; Hyland, J.; Zimmermann, J.; McCarthy, S. Review: Trends for meat, milk and egg consumption for the next decades and the role played by livestock systems in the global production of proteins. Animal 2021, 15, 100287. [Google Scholar] [CrossRef]
- Guyomarc’h, F.; Arvisenet, G.; Bouhallab, S.; Canon, F.; Deutsch, S.; Drigon, V.; Dupont, D.; Nau, F.; Pezennec, S.; Thierry, A.; et al. Mixing milk, egg and plant resources to obtain safe and tasty foods with environmental and health benefits. Trends Food Sci. Technol. 2021, 108, 119–132. [Google Scholar] [CrossRef]
- Abeyrathne, E.; Lee, H.; Ham, J.; Ahn, D. Separation of ovotransferrin from chicken egg white without using organic solvents. Poult. Sci. 2013, 92, 1091–1097. [Google Scholar] [CrossRef]
- Ko, K.; Ahn, D. An Economic and Simple Purification Procedure for the Large-Scale Production of Ovotransferrin from Egg White. Poult. Sci. 2008, 87, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Renkema, J.M.S.; Gruppen, H.; van Vliet, T. Influence of pH and Ionic Strength on Heat-Induced Formation and Rheological Properties of Soy Protein Gels in Relation to Denaturation and Their Protein Compositions. J. Agric. Food Chem. 2002, 50, 6064–6071. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Oey, I.; Bremer, P.; Carne, A.; Silcock, P. Effects of pH, temperature and pulsed electric fields on the turbidity and protein aggregation of ovomucin-depleted egg white. Food Res. Int. 2017, 91, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Anema, S.G. Chapter 9-The whey proteins in milk: Thermal denaturation, physical interactions, and effects on the functional properties of milk. In Milk Proteins, 3rd ed.; Boland, M., Singh, H., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 325–384. [Google Scholar] [CrossRef]
- Dissanayake, M.; Ramchandran, L.; Donkor, O.N.; Vasiljevic, T. Denaturation of whey proteins as a function of heat, pH and protein concentration. Int. Dairy J. 2013, 31, 93–99. [Google Scholar] [CrossRef]
- Franzoi, M.; Costa, A.; Vigolo, V.; Penasa, M.; De Marchi, M. Effect of pasteurization on coagulation properties of bovine milk and the role of major composition traits and protein fractions. J. Food Compos. Anal. 2022, 114, 104808. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Separation of ovotransferrin and ovomucoid from chicken egg white. Poult. Sci. 2014, 93, 1010–1017. [Google Scholar] [CrossRef]
- Zeng, Q.; Zeng, W.; Jin, Y.; Sheng, L. Construction and evaluation of ovalbumin-pullulan nanogels as a potential delivery carrier for curcumin. Food Chem. 2022, 367, 130716. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Q.; Wu, Y.; Wang, G.; Geng, F.; Song, H.; Luo, P.; Huang, Q. Effect of ball milling-assisted glycosylation modification on the structure and foaming property of egg white protein. J. Food Sci. 2022, 87, 3117–3128. [Google Scholar] [CrossRef]
- Li, P.; Jin, Y.; Sheng, L. Impact of microwave assisted phosphorylation on the physicochemistry and rehydration behaviour of egg white powder. Food Hydrocoll. 2020, 100, 105380. [Google Scholar] [CrossRef]
- Jin, H.; Li, P.; Jin, Y.; Sheng, L. Effect of sodium tripolyphosphate on the interaction and aggregation behavior of ovalbumin-lysozyme complex. Food Chem. 2021, 352, 129457. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, L.; Wu, Y.; Huang, X.; Luo, P. Mechanism of differences in characteristics of thick/thin egg whites during storage: Physicochemical, functional and molecular structure characteristics analysis. Food Chem. 2021, 369, 130828. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, Q.; Liu, Y.; Cai, Z. Enhancing the resistance of anthocyanins to environmental stress by constructing ovalbumin-propylene glycol alginate nanocarriers with novel configurations. Food Hydrocoll. 2021, 118, 106668. [Google Scholar] [CrossRef]
- Flanagan, S.E.; Malanowski, A.J.; Kizilay, E.; Seeman, D.; Dubin, P.L.; Donato-Capel, L.; Bovetto, L.; Schmitt, C. Complex equilibria, speciation, and heteroprotein coacervation of lactoferrin and β-lactoglobulin. Langmuir 2015, 31, 1776–1783. [Google Scholar] [CrossRef]
- Muralidhara, B.K.; Hirose, M. Structural and functional consequences of removal of the interdomain disulfide bridge from the isolated C-lobe of ovotransferrin. Protein Sci. 2000, 9, 1567–1575. [Google Scholar] [CrossRef] [Green Version]
- Anderson, B.F.; Baker, H.M.; Dodson, E.J.; Norris, G.E.; Rumball, S.V.; Waters, J.M.; Baker, E.N. Structure of human lactoferrin at 3.2 A resolution. Proc. Natl. Acad. Sci. USA 1987, 84, 1769–1773. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wu, W.; Deng, Z.; Zhang, S.; Guan, W. Improved thermostability of lipase Lip2 from Yarrowia lipolytica through disulfide bond design for preparation of medium-long-medium structured lipids. LWT 2022, 166, 113786. [Google Scholar] [CrossRef]
- Williams, J.; Elleman, T.C.; Barrykingston, I.; Wilkins, A.G.; Kuhn, K.A. The primary structure of hen ovotransferrin. Eur. J. Biochem. 1982, 122, 297–303. [Google Scholar] [CrossRef]
- Gnanesh Kumar, B.S.; Mattad, S. Comprehensive analysis of lactoferrin N-glycans with site-specificity from bovine colostrum using specific proteases and RP-UHPLC-MS/MS. Int. Dairy J. 2021, 119, 104999. [Google Scholar] [CrossRef]
- Metz-Boutigue, M.; Jolles, J.; Mazurier, J.; Schoentgen, F.; Legrand, D.; Spik, G.; Montreuil, J.; Jolles, P. Human lactotransferrin: Amino acid sequence and structural comparisons with other transferrins. Eur. J. Biochem. 1984, 145, 659–676. [Google Scholar] [CrossRef]
- Kurokawa, H.; Mikami, B.; Hirose, M. Crystal structure of diferric hen ovotransferrin at 2.4 A resolution. J. Mol. Biol. 1995, 254, 196–207. [Google Scholar] [CrossRef]
- Wu, H.M.; Church, F.C. Arginine 25 and Arginine 28 of lactoferrin are critical for effective heparin neutralization in blood. Arch. Biochem. Biophys. 2003, 412, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Kraan, M.; Nazmi, K.; Teeken, A.; Groenink, J.; Hof, W.; Veerman, E.; Bolscher, J.; Amerongen, A. Lactoferrampin, an antimicrobial peptide of bovine lactoferrin, exerts its candidacidal activity by a cluster of positively charged residues at the C-terminus in combination with a helix-facilitating N-terminal part. Biol. Chem. 2005, 386, 137–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Z.; Zhang, M.; Ma, M. Emulsifying properties of ovalbumin: Improvement and mechanism by phosphorylation in the presence of sodium tripolyphosphate. Food Hydrocoll. 2016, 60, 29–37. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Ruan, J.; Wang, Y.; Li, X.; Xu, M.; Zhao, J.; Lin, H.; Liu, P.; Wang, Z.; et al. Effects of lysine and arginine addition combined with high-pressure microfluidization treatment on the structure, solubility, and stability of pork myofibrillar proteins. LWT 2022, 172, 114190. [Google Scholar] [CrossRef]
- Yu, Z.; Reid, J.C.; Yang, Y.P. Utilizing Dynamic Light Scattering as a Process Analytical Technology for Protein Folding and Aggregation Monitoring in Vaccine Manufacturing. J. Pharm. Sci. 2013, 102, 4284–4290. [Google Scholar] [CrossRef]
- Lan, H.; Liu, H.; Ye, Y.; Yin, Z. The Role of Surface Properties on Protein Aggregation Behavior in Aqueous Solution of Different pH Values. AAPS PharmSciTech 2020, 21, 122. [Google Scholar] [CrossRef] [PubMed]
- Matsudomi, N.; Takasaki, M.; Kobayashi, K. Heat-induced Aggregation of Lysozyme with Ovotransferrin. Agric. Biol. Chem. 1991, 55, 1651–1653. [Google Scholar] [CrossRef]
- Tang, S.; Li, J.; Huang, G.; Yan, L. Predicting Protein Surface Property with its Surface Hydrophobicity. Protein Pept. Lett. 2021, 28, 938–944. [Google Scholar] [CrossRef]
- Ota, C.; Tanaka, S.I.; Takano, K. Revisiting the Rate-Limiting Step of the ANS-Protein Binding at the Protein Surface and Inside the Hydrophobic Cavity. Molecules 2021, 26, 420. [Google Scholar] [CrossRef]
- Moore, S.A.; Anderson, B.F.; Groom, C.R.; Haridas, M.; Baker, E.N. Three-dimensional structure of diferric bovine lactoferrin at 2.8 Å resolution11Edited by D. Rees. J. Mol. Biol. 1997, 274, 222–236. [Google Scholar] [CrossRef]
- Großmann, G.; Neu, M.; Pantos, E.; Townes-Andrews, E.; Schwab, F.; Evans, R.W.; Lindley, P.F.; Thies, W.G.; Appel, H.; Hasnain, S.S. X-ray small angle solution scattering studies reveal metal-induced conformational changes in transferrins. J. Inorg. Biochem. 1991, 43, 135. [Google Scholar] [CrossRef]
- Pena-Francesch, A.; Jung, H.; Demirel, M.C.; Sitti, M. Biosynthetic self-healing materials for soft machines. Nat. Mater. 2020, 19, 1230–1235. [Google Scholar] [CrossRef]
- He, Z.; Cheng, H.; Olanya, M.; Uknalis, J.; Zhang, X.; Koplitz, B.; He, J. Surface Characterization of Cottonseed Meal Products by SEM, SEM-EDS, XRD and XPS Analysis. J. Mater. Sci. Res. 2017, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zhu, H.; Zhang, B.; Chen, J.; Ao, Q.; Wang, X. XRD, SEM, and XPS Analysis of Soybean Protein Powders Obtained Through Extraction Involving Reverse Micelles. J. Am. Oil Chem. Soc. 2015, 92, 975–983. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Zhu, Q.; Chen, F.; Zhao, X.; Ao, Q. Determination of the domain structure of the 7S and 11S globulins from soy proteins by XRD and FTIR. J. Sci. Food Agric. 2013, 93, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Voronin, G.; Harte, F. Differential Scanning Calorimetry. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Academic Press: Oxford, UK, 2022; pp. 348–355. [Google Scholar] [CrossRef]
- Castillo, E.; Pérez, M.; Franco, I.; Calvo, M.; Sánchez, L. Kinetic and thermodynamic parameters for heat denaturation of human recombinant lactoferrin from rice. Biochem. Cell Biol. 2012, 90, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Lei, D.; Ma, X. Effect of enzymatic glycosylation on the structure and properties of wheat gluten protein fibers. J. Eng. Fibers Fabr. 2021, 16, 179–208. [Google Scholar] [CrossRef]
- Pereira, R.N.; Teixeira, J.A.; Vicente, A.A. Exploring the denaturation of whey proteins upon application of moderate electric fields: A kinetic and thermodynamic study. J. Agric. Food Chem. 2011, 59, 11589–11597. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.; Singh, H.; Creamer, L.K. Heat-induced interactions of beta-lactoglobulin A and kappa-casein B in a model system. J. Dairy Res. 2003, 70, 61. [Google Scholar] [CrossRef]
- Chehín, R.; Iloro, I.; Marcos, M.J.; Villar, E.; Shnyrov, V.L.; Arrondo, J.L.R. Thermal and pH-Induced Conformational Changes of a β-Sheet Protein Monitored by Infrared Spectroscopy. Biochemistry 1999, 38, 1525–1530. [Google Scholar] [CrossRef]
- Pace, C.N.; Grimsley, G.R.; Scholtz, J.M. Protein ionizable groups: pK values and their contribution to protein stability and solubility. J. Biol. Chem. 2009, 284, 13285–13289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, N.; Singh, A.; Singh, P.K.; Tyagi, T.K.; Pandey, S.; Shin, K.; Kaur, P.; Sharma, S.; Singh, T.P. Structure of iron saturated C-lobe of bovine lactoferrin at p H 6.8 indicates a weakening of iron coordination. Proteins Struct. Funct. Bioinform. 2016, 84, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.T.; Ma, C.Y. Thermal properties of Phaseolus angularis (red bean) globulin. Food Chem. 2001, 73, 453–460. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Dynamic oscillatory rheological measurement and thermal properties of pea protein extracted by salt method: Effect of pH and NaCl. J. Food Eng. 2011, 105, 577–582. [Google Scholar] [CrossRef]
- Baker, E.N.; Anderson, B.F.; Baker, H.M.; Haridas, M.; Jameson, G.B.; Norris, G.E.; Rumball, S.V.; Smith, C.A. Structure, function and flexibility of human lactoferrin. Int. J. Biol. Macromol. 1991, 13, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Seyrek, E.; Dubin, P.L.; Tribet, C.; Gamble, E.A. Ionic Strength Dependence of Protein-Polyelectrolyte Interactions. Biomacromolecules 2003, 4, 273–282. [Google Scholar] [CrossRef]
- Tanger, C.; Müller, M.; Andlinger, D.; Kulozik, U. Influence of pH and ionic strength on the thermal gelation behaviour of pea protein. Food Hydrocoll. 2022, 123, 106903. [Google Scholar] [CrossRef]
- Lewis, M. Chapter 4-Food Acidity, pH and Redox Potential. In Food Process Engineering Principles and Data; Lewis, M., Ed.; Woodhead: Sawston, UK, 2023; pp. 21–27. [Google Scholar] [CrossRef]
- Piumetti, M. Structure of Proteins. In Molecular Dynamics and Complexity in Catalysis and Biocatalysis; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–21. [Google Scholar] [CrossRef]
- Baird, G.; Farrell, C.; Cheung, J.; Semple, A.; Blue, J.; Ahl, P.L. FTIR Spectroscopy Detects Intermolecular β-Sheet Formation Above the High Temperature Tm for Two Monoclonal Antibodies. Protein J. 2020, 39, 318–327. [Google Scholar] [CrossRef]
- Narayanan, C.; Dias, C. Roles of Hydrophobic Interactions and Hydrogen Bonds in Beta-Sheet Formation. J. Chem. Phys. 2013, 139, 115103. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Li, L.; Xu, D.; Sheng, B.; Qin, D.; Chen, J.; Li, B.; Zhang, X. Application of ultrasound pretreatment and glycation in regulating the heat-induced amyloid-like aggregation of β-lactoglobulin. Food Hydrocoll. 2018, 80, 122–129. [Google Scholar] [CrossRef]
- Hwang, S.; Shao, Q.; Williams, H.; Hilty, C.; Gao, Y.Q. Methanol strengthens hydrogen bonds and weakens hydrophobic interactions in proteins: A combined molecular dynamics and NMR study. J. Phys. Chem. B 2011, 115, 6653–6660. [Google Scholar] [CrossRef]
- Cheng, W.; Ma, J.; Wang, S.; Lou, R.; Wu, S.; He, J.; Kang, H.; Liu, L.; Xiao, F. Interaction mechanism between resveratrol and ovalbumin based on fluorescence spectroscopy and molecular dynamic simulation. LWT 2021, 146, 111455. [Google Scholar] [CrossRef]
- Jahanban Esfahlan, A.; Panahi Azar, V.; Sajedi, S.; Case, D. Spectroscopic and molecular docking studies on the interaction between N-Acetyl Cysteine (NAC) and bovine serum albumin. Biopolymers 2015, 103, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Keerati-u-rai, M.; Miriani, M.; Iametti, S.; Bonomi, F.; Corredig, M. Structural changes of soy proteins at the oil-water interface studied by fluorescence spectroscopy. Colloids Surf. B Biointerfaces 2012, 93, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Bakerheather, M.; Bakeredward, N. A structural perspective on lactoferrin function. Biochem. Cell Biol. Biochim. Biol. Cell. 2012, 90, 320–328. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, L. Research on Turbidity and Surface Hydrophobicity of Milk Protein at Different Conditions. J. Phys. Conf. Ser. 2021, 1893, 012009. [Google Scholar] [CrossRef]
- Oki, S.; Iwashita, K.; Kimura, M.; Kano, H.; Shiraki, K. Mechanism of co-aggregation in a protein mixture with small additives. Int. J. Biol. Macromol. 2018, 107, 1428–1437. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Huang, C.; Cai, B.; Guo, F.; Chen, L.; Feng, X.; Ma, M. Improved thermal tolerance of ovotransferrin against pasteurization by phosphorylation. Food Chem. 2023, 405, 135019. [Google Scholar] [CrossRef]
- Yang, W.; Xu, C.; Liu, F.; Yuan, F.; Gao, Y. Native and Thermally Modified Protein–Polyphenol Coassemblies: Lactoferrin-Based Nanoparticles and Submicrometer Particles as Protective Vehicles for (-)-Epigallocatechin-3-gallate. J. Agric. Food Chem. 2014, 62, 10816–10827. [Google Scholar] [CrossRef]
- Yang, W.; Qu, X.; Deng, C.; Dai, L.; Zhou, H.; Xu, G.; Li, B.; Yulia, N.; Liu, C. Heat sensitive protein-heat stable protein interaction: Synergistic enhancement in the thermal co-aggregation and gelation of lactoferrin and α-lactalbumin. Food Res. Int. 2021, 142, 110179. [Google Scholar] [CrossRef]
- Hirose, M. Molten globule state of food proteins. Trends Food Sci. Technol. 1993, 4, 48–51. [Google Scholar] [CrossRef]









| Sample | Sulfhydryl Content | |
|---|---|---|
| Free Sulfhydryl (μmol/g) | Total Sulfhydryl (μmol/g) | |
| OVT | ND | 196.10 ± 1.89 b |
| BLF | ND | 202.01 ± 3.07 a |
| HLF | ND | 204.32 ± 3.44 a |
| Sample | 2θ | Intensity | Area | Distance (Å) |
|---|---|---|---|---|
| OVT | 9.82 ± 0.53 c | 449 ± 35 c | 95,778 ± 2631 d | 9.02 ± 0.16 b |
| 21.20 ± 0.34 b | 1235 ± 76 a | 519,901 ± 5372 a | 4.19 ± 0.24 c | |
| BLF | 8.82 ± 0.19 d | 202 ± 43 d | 32,602 ± 2132 e | 10.01 ± 0.25 a |
| 21.02 ± 0.28 b | 816 ± 65 b | 346,868 ± 4627 c | 4.22 ± 0.33 c | |
| HLF | 9.06 ± 0.22 d | 223 ± 28 d | 31,396 ± 1847 e | 9.76 ± 0.36 a |
| 22.59 ± 0.55 a | 864 ± 63 b | 386,502 ± 4784 b | 3.93 ± 0.28 c |
| Sample | Treatment | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) |
|---|---|---|---|---|---|
| OVT | Nature/5.0 | 20.55 ± 0.07 c | 21.80 ± 0.85 g | 21.85 ± 0.78 c | 35.80 ± 0.97 a |
| Nature/7.0 | 20.35 ± 1.91 c | 19.85 ± 5.73 g | 23.20 ± 3.25 bc | 36.60 ± 0.57 a | |
| Nature/9.0 | 19.50 ± 1.56 cd | 26.30 ± 1.84 e | 20.10 ± 2.26 c | 34.15 ± 1.91 ab | |
| 70 °C/5.0 | 8.75 ± 0.35 f | 53.85 ± 1.20 b | 8.20 ± 0.14 f | 29.10 ± 0.99 c | |
| 70 °C/7.0 | 15.4 ± 0.42 e | 36.80 ± 0.57 c | 15.35 ± 0.92 e | 32.45 ± 0.64 b | |
| 70 °C/9.0 | 13.95 ± 0.07 e | 37.50 ± 1.98 c | 14.30 ± 0.85 e | 34.25 ± 1.20 ab | |
| BLF | Nature /5.0 | 17.45 ± 2.62 d | 33.90 ± 2.91 c | 18.65 ± 1.30 d | 30.00 ± 0.99 c |
| Nature /7.0 | 20.05 ± 0.91 c | 26.35 ± 0.35 e | 21.70 ± 0.71 c | 31.90 ± 0.28 c | |
| Nature /9.0 | 19.45 ± 0.35 cd | 29.20 ± 0.57 d | 21.35 ± 0.07 c | 29.95 ± 0.92 c | |
| 70 °C/5.0 | 17.95 ± 0.49 d | 33.50 ± 0.57 c | 18.75 ± 0.35 d | 29.85 ± 1.34 c | |
| 70 °C/7.0 | 6.65 ± 0.78 f | 61.40 ± 1.56 a | 6.75 ± 1.48 f | 25.15 ± 0.78 d | |
| 70 °C/9.0 | 7.80 ± 0.57 f | 54.85 ± 2.90 b | 7.90 ± 1.27 f | 29.45 ± 0.92 c | |
| HLF | Nature/5.0 | 26.35 ± 1.34 a | 8.60 ± 5.52 h | 30.35 ± 2.90 a | 34.70 ± 1.27 ab |
| Nature/7.0 | 28.45 ± 1.48 a | 4.50 ± 1.56 i | 32.65 ± 1.77 a | 34.40 ± 1.70 ab | |
| Nature/9.0 | 27.05 ± 0.92 a | 9.45 ± 2.47 h | 30.55 ± 1.48 a | 32.90 ± 0.14 bc | |
| 70 °C/5.0 | 23.95 ± 0.64 b | 17.50 ± 4.24 g | 26.45 ± 1.77 b | 32.10 ± 1.84 bc | |
| 70 °C/7.0 | 26.30 ± 0.42 a | 10.20 ± 2.12 h | 30.10 ± 1.13 a | 33.40 ± 0.57 bc | |
| 70 °C/9.0 | 22.05 ± 0.21 b | 24.25 ± 0.64 f | 22.90 ± 0.42 c | 30.75 ± 0.07 c |
| Sample | Turbidity (%) | |||||
|---|---|---|---|---|---|---|
| Nature/5.0 | Nature/7.0 | Nature/9.0 | 70 °C/5.0 | 70 °C/7.0 | 70 °C/9.0 | |
| OVT | 0.34 ± 0.16 c | 1.71 ± 0.80 c | 2.04 ± 1.09 c | 56.72 ± 1.32 a | 30.98 ± 0.23 b | 4.83 ± 0.77 c |
| BLF | 2.28 ± 0.18 d | 3.06 ± 0.43 d | 2.61 ± 0.07 d | 7.85 ± 0.15 c | 59.53 ± 2.38 a | 12.80 ± 0.71 b |
| HLF | 4.28 ± 0.31 d | 5.27 ± 0.15 d | 5.92 ± 0.46 d | 45.76 ± 2.62 a | 35.66 ± 0.14 c | 39.87 ± 1.55 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Q.; Liu, Y.; Sun, J.; Jin, Y. Providing New Insights on the Molecular Properties and Thermal Stability of Ovotransferrin and Lactoferrin. Foods 2023, 12, 532. https://doi.org/10.3390/foods12030532
Zeng Q, Liu Y, Sun J, Jin Y. Providing New Insights on the Molecular Properties and Thermal Stability of Ovotransferrin and Lactoferrin. Foods. 2023; 12(3):532. https://doi.org/10.3390/foods12030532
Chicago/Turabian StyleZeng, Qi, Yaping Liu, Jing Sun, and Yongguo Jin. 2023. "Providing New Insights on the Molecular Properties and Thermal Stability of Ovotransferrin and Lactoferrin" Foods 12, no. 3: 532. https://doi.org/10.3390/foods12030532