Improvement of Sinapine Extraction from Mustard Seed Meal by Application of Emerging Technologies
Abstract
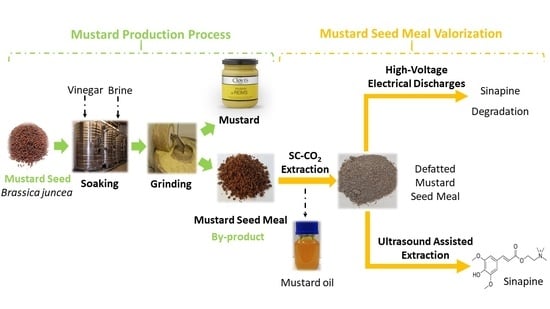
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Conventional Extraction of the Sinapine
2.3. Extraction Technologies
2.3.1. High-Voltage Electrical Discharges (HVEDs)
2.3.2. Supercritical CO2
2.3.3. Ultrasound-Assisted Extraction
2.4. Kinetic Modeling of the Extraction of Sinapine
2.5. Analytical Measurements
2.5.1. Quantification of Sinapine by UHPLC
2.5.2. Gas Chromatography–Tandem Mass Spectrometry (GC–MS/MS)
2.5.3. Environmental Scanning Electron Microscopy (ESEM)
2.6. Statistical Analysis
3. Results and Discussions
3.1. Effect of the HVEDs on the Sinapine Extraction
3.2. Effect of the SC-CO2 Extraction as Pretreatment Technology on the Sinapine Extraction
3.3. Effect of the Ultrasound-Assisted Extraction
3.4. Environmental Scanning Electron Microscopy (ESEM) Observations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reungoat, V. Développement d’un Procédé D’obtention D’acide Sinapique à Partir de Son de Moutarde et de Tourteau de Colza via La Mise En Œuvre de Procédés D’extraction par Solvant, D’hydrolyse Enzymatique et de Purification Par Contacteur Membranaire. Ph.D. Thesis, AgroParisTech, Paris, France, 2022. [Google Scholar]
- Sehwag, S.; Swati; Das, M. A Brief Overview: Present Status on Utilization of Mustard Oil and Cake. Indian J. Tradit. Knowl. 2015, 14, 244–250. [Google Scholar]
- Badey, L.; Torrijos, M.; Sousbie, P.; Pouech, P.; Bosque, F. La valorisation des coproduits de l’huilerie par méthanisation. Ol. Corps Gras Lipides 2012, 19, 358–369. [Google Scholar] [CrossRef]
- Favela-González, K.M.; Hernández-Almanza, A.Y.; De la Fuente-Salcido, N.M. The Value of Bioactive Compounds of Cruciferous Vegetables (Brassica) as Antimicrobials and Antioxidants: A Review. J. Food Biochem. 2020, 44, e13414. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.P.T.; Stewart, J.; Lopez, M.; Ioannou, I.; Allais, F. Glucosinolates: Natural Occurrence, Biosynthesis, Accessibility, Isolation, Structures, and Biological Activities. Molecules 2020, 25, 4537. [Google Scholar] [CrossRef]
- Arena, K.; Cacciola, F.; Dugo, L.; Dugo, P.; Mondello, L. Determination of the Metabolite Content of Brassica Juncea Cultivars Using Comprehensive Two-Dimensional Liquid Chromatography Coupled with a Photodiode Array and Mass Spectrometry Detection. Molecules 2020, 25, 1235. [Google Scholar] [CrossRef] [Green Version]
- Laguna, O.; Barakat, A.; Alhamada, H.; Durand, E.; Baréa, B.; Fine, F.; Villeneuve, P.; Citeau, M.; Dauguet, S.; Lecomte, J. Production of Proteins and Phenolic Compounds Enriched Fractions from Rapeseed and Sunflower Meals by Dry Fractionation Processes. Ind. Crops Prod. 2018, 118, 160–172. [Google Scholar] [CrossRef]
- Naczk, M.; Amarowicz, R.; Sullivan, A.; Shahidi, F. Current Research Developments on Polyphenolics of Rapeseed/Canola: A Review. Food Chem. 1998, 62, 489–502. [Google Scholar] [CrossRef]
- Reungoat, V.; Gaudin, M.; Flourat, A.L.; Isidore, E.; Mouterde, L.M.M.; Allais, F.; Ducatel, H.; Ioannou, I. Optimization of an Ethanol/Water-Based Sinapine Extraction from Mustard Bran Using Response Surface Methodology. Food Bioprod. Process. 2020, 122, 322–331. [Google Scholar] [CrossRef]
- Khattab, R.; Eskin, M.; Aliani, M.; Thiyam, U. Determination of Sinapic Acid Derivatives in Canola Extracts Using High-Performance Liquid Chromatography. J. Am. Oil Chem. Soc. 2010, 87, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.P.T.; Stewart, J.D.; Ioannou, I.; Allais, F. Sinapic Acid and Sinapate Esters in Brassica: Innate Accumulation, Biosynthesis, Accessibility via Chemical Synthesis or Recovery From Biomass, and Biological Activities. Front. Chem. 2021, 9. [Google Scholar] [CrossRef]
- Abramovič, H. Chapter 93—Antioxidant Properties of Hydroxycinnamic Acid Derivatives: A Focus on Biochemistry, Physicochemical Parameters, Reactive Species, and Biomolecular Interactions. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 843–852. ISBN 978-0-12-409517-5. [Google Scholar]
- Chadni, M.; Flourat, A.L.; Reungoat, V.; Mouterde, L.M.M.; Allais, F.; Ioannou, I. Selective Extraction of Sinapic Acid Derivatives from Mustard Seed Meal by Acting on PH: Toward a High Antioxidant Activity Rich Extract. Molecules 2021, 26, 212. [Google Scholar] [CrossRef] [PubMed]
- Dubie, J.; Stancik, A.; Morra, M.; Nindo, C. Antioxidant Extraction from Mustard (Brassica Juncea) Seed Meal Using High-Intensity Ultrasound. J. Food Sci. 2013, 78, E542–E548. [Google Scholar] [CrossRef] [PubMed]
- Matthäus, B. Antioxidant Activity of Extracts Obtained from Residues of Different Oilseeds. J. Agric. Food Chem. 2002, 50, 3444–3452. [Google Scholar] [CrossRef]
- Fahmi, R. Antioxidant and Antibacterial Properties of Endogenous Phenolic Compounds from Commercial Mustard Products. Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 2016. [Google Scholar]
- Yuan, L.; Scanlon, M.G.; Eskin, N.A.M.; Thiyam-Hollander, U.; Aachary, A.A. Effect of Pretreatments and Endo-1,4-β-Xylanase Hydrolysis of Canola Meal and Mustard Bran for Production of Oligosaccharides. Appl. Biochem. Biotechnol. 2015, 175, 194–208. [Google Scholar] [CrossRef]
- Seal, C.E.; Kranner, I.; Pritchard, H.W. Quantification of Seed Oil from Species with Varying Oil Content Using Supercritical Fluid Extraction. Phytochem. Anal. PCA 2008, 19, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Barthet, V.J.; Daun, J.K. An Evaluation of Supercritical Fluid Extraction as an Analytical Tool to Determine Fat in Canola, Flax, Solin, and Mustard. J. Am. Oil Chem. Soc. 2002, 79, 245–251. [Google Scholar] [CrossRef]
- Hernández-Corroto, E.; Boussetta, N.; Marina, M.L.; García, M.C.; Vorobiev, E. High Voltage Electrical Discharges Followed by Deep Eutectic Solvents Extraction for the Valorization of Pomegranate Seeds (Punica granatum L.). Innov. Food Sci. Emerg. Technol. 2022, 79, 103055. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E.; Reess, T.; De Ferron, A.; Pecastaing, L.; Ruscassié, R.; Lanoisellé, J.-L. Scale-up of High Voltage Electrical Discharges for Polyphenols Extraction from Grape Pomace: Effect of the Dynamic Shock Waves. Innov. Food Sci. Emerg. Technol. 2012, 16, 129–136. [Google Scholar] [CrossRef]
- Brahim, M.; Checa Fernandez, B.L.; Regnier, O.; Boussetta, N.; Grimi, N.; Sarazin, C.; Husson, E.; Vorobiev, E.; Brosse, N. Impact of Ultrasounds and High Voltage Electrical Discharges on Physico-Chemical Properties of Rapeseed Straw’s Lignin and Pulps. Bioresour. Technol. 2017, 237, 11–19. [Google Scholar] [CrossRef]
- El Kantar, S.; Boussetta, N.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. High Voltage Electrical Discharges Combined with Enzymatic Hydrolysis for Extraction of Polyphenols and Fermentable Sugars from Orange Peels. Food Res. Int. 2018, 107, 755–762. [Google Scholar] [CrossRef]
- Rajha, H.N.; Abi-Khattar, A.-M.; El Kantar, S.; Boussetta, N.; Lebovka, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Comparison of Aqueous Extraction Efficiency and Biological Activities of Polyphenols from Pomegranate Peels Assisted by Infrared, Ultrasound, Pulsed Electric Fields and High-Voltage Electrical Discharges. Innov. Food Sci. Emerg. Technol. 2019, 58, 102212. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Boussetta, N.; Tessaro, I.C.; Marczak, L.D.F.; Vorobiev, E. Application of Pulsed Electric Fields and High Voltage Electrical Discharges for Oil Extraction from Sesame Seeds. J. Food Eng. 2015, 153, 20–27. [Google Scholar] [CrossRef]
- Boussetta, N.; Lesaint, O.; Vorobiev, E. A Study of Mechanisms Involved during the Extraction of Polyphenols from Grape Seeds by Pulsed Electrical Discharges. Innov. Food Sci. Emerg. Technol. 2013, 19, 124–132. [Google Scholar] [CrossRef]
- Hebert, M.; Mhemdi, H.; Vorobiev, E. Selective and Eco-Friendly Recovery of Glucosinolates from Mustard Seeds (Brassica juncea) Using Process Optimization and Innovative Pretreatment (High Voltage Electrical Discharges). Food Bioprod. Process. 2020, 124, 11–23. [Google Scholar] [CrossRef]
- Yu, J.; Wang, J.; Liu, C.; Liu, Z.; Wang, Q. Application of Response Surface Methodology to Optimise Supercritical Carbon Dioxide Extraction of Oil from Rapeseed (Brassica napus L.). Int. J. Food Sci. Technol. 2012, 47, 1115–1121. [Google Scholar] [CrossRef]
- Flourat, A.L.; Willig, G.; Teixeira, A.R.S.; Allais, F. Eco-Friendly Extraction of Sinapine From Residues of Mustard Production. Front. Sustain. Food Syst. 2019, 3, 12. [Google Scholar] [CrossRef]
- Ashokkumar, M. Applications of Ultrasound in Food and Bioprocessing. Ultrason. Sonochem. 2015, 25, 17–23. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Tułodziecka, A. Application of Response Surface Methodology to Optimize Ultrasound-Assisted Extraction of Total Antioxidants from Brassica Napus Cultivars. Eur. J. Lipid Sci. Technol. 2015, 117, 491–502. [Google Scholar] [CrossRef]
- Terpinc, P.; Čeh, B.; Ulrih, N.P.; Abramovič, H. Studies of the Correlation between Antioxidant Properties and the Total Phenolic Content of Different Oil Cake Extracts. Ind. Crops Prod. 2012, 39, 210–217. [Google Scholar] [CrossRef]
- Teh, S.-S.; Birch, E.J. Effect of Ultrasonic Treatment on the Polyphenol Content and Antioxidant Capacity of Extract from Defatted Hemp, Flax and Canola Seed Cakes. Ultrason. Sonochem. 2014, 21, 346–353. [Google Scholar] [CrossRef]
- Fattori, M.; Bulley, N.R.; Meisen, A. Carbon Dioxide Extraction of Canola Seed: Oil Solubility and Effect of Seed Treatment. J. Am. Oil Chem. Soc. 1988, 65, 968–974. [Google Scholar] [CrossRef]
- Pederssetti, M.M.; Palú, F.; da Silva, E.A.; Rohling, J.H.; Cardozo-Filho, L.; Dariva, C. Extraction of Canola Seed (Brassica napus) Oil Using Compressed Propane and Supercritical Carbon Dioxide. J. Food Eng. 2011, 102, 189–196. [Google Scholar] [CrossRef]
- Taniguchi, M.; Nomura, R.; Kijima, I.; Kobayashi, T. Preparation of Defatted Mustard by Extraction with Supercritical Carbon Dioxide. Agric. Biol. Chem. 1987, 51, 413–417. [Google Scholar] [CrossRef] [Green Version]
- Chadni, M.; Isidore, E.; Diemer, E.; Ouguir, O.; Brunois, F.; Catteau, R.; Cassan, L.; Ioannou, I. Optimization of Extraction Conditions to Improve Chlorogenic Acid Content and Antioxidant Activity of Extracts from Forced Witloof Chicory Roots. Foods 2022, 11, 1217. [Google Scholar] [CrossRef]
- Jurinjak Tušek, A.; Benković, M.; Belščak Cvitanović, A.; Valinger, D.; Jurina, T.; Gajdoš Kljusurić, J. Kinetics and Thermodynamics of the Solid-Liquid Extraction Process of Total Polyphenols, Antioxidants and Extraction Yield from Asteraceae Plants. Ind. Crops Prod. 2016, 91, 205–214. [Google Scholar] [CrossRef]
- Dron, J.; Linke, R.; Rosenberg, E.; Schreiner, M. Trimethylsulfonium Hydroxide as Derivatization Reagent for the Chemical Investigation of Drying Oils in Works of Art by Gas Chromatography. J. Chromatogr. A 2004, 1047, 111–116. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Boussetta, N.; Lanoisellé, J.-L.; Bedel-Cloutour, C.; Vorobiev, E. Extraction of Soluble Matter from Grape Pomace by High Voltage Electrical Discharges for Polyphenol Recovery: Effect of Sulphur Dioxide and Thermal Treatments. J. Food Eng. 2009, 95, 192–198. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E.; Deloison, V.; Pochez, F.; Falcimaigne-Cordin, A.; Lanoisellé, J.-L. Valorisation of Grape Pomace by the Extraction of Phenolic Antioxidants: Application of High Voltage Electrical Discharges. Food Chem. 2011, 128, 364–370. [Google Scholar] [CrossRef]
- Bogomaz, A.A.; Goryachev, V.L.; Remennyi, A.S.; Rutberg, F.G. The effectiveness of a pulsed electrical discharge in decontaminating water. Sov. Tech. Phys. Lett. 1991, 17, 448–449. [Google Scholar]
- Chen, Y.-S.; Zhang, X.-S.; Dai, Y.-C.; Yuan, W.-K. Pulsed High-Voltage Discharge Plasma for Degradation of Phenol in Aqueous Solution. Sep. Purif. Technol. 2004, 34, 5–12. [Google Scholar] [CrossRef]
- Tyśkiewicz, K.; Konkol, M.; Rój, E. The Application of Supercritical Fluid Extraction in Phenolic Compounds Isolation from Natural Plant Materials. Mol. J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Zhao, J.; Li, H.; Song, F. Supercritical Fluid Extraction of Total Flavonoids from Leaves of Acanthopanax Senticosus Harms* *Supported by the Natural Science and Technology Foundation of Jilin Province(No. 20020637-1). Chem. Res. Chin. Univ. 2007, 23, 233–236. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]







| SAT (%) | MONOSAT (%) | POLYSAT (%) | SAT/UNSAT Ratio | Omega 3/ Omega 6 Ratio | SAT/ Omega 6 Ratio | SAT/ Omega 3 Ratio |
|---|---|---|---|---|---|---|
| 17.87 | 47.71 | 34.40 | 0.22 | 0.67 | 0.90 | 1.34 |
| Parameter Values | Response | |||
|---|---|---|---|---|
| Run | Temperature (°C) | Ethanol (%) | Amplitude (%) | Sinapine (mg/g DM) |
| 1 | 25 | 0 | 50 | 0.15 |
| 2 | 75 | 0 | 50 | 3.71 |
| 3 | 25 | 70 | 50 | 2.68 |
| 4 | 75 | 70 | 50 | 6.37 |
| 5 | 25 | 35 | 0 | 0.00 |
| 6 | 75 | 35 | 0 | 3.15 |
| 7 | 25 | 35 | 100 | 2.65 |
| 8 | 75 | 35 | 100 | 4.39 |
| 9 | 50 | 0 | 0 | 2.19 |
| 10 | 50 | 70 | 0 | 4.18 |
| 11 | 50 | 0 | 100 | 3.21 |
| 12 | 50 | 70 | 100 | 5.2 |
| 13 | 50 | 35 | 50 | 3.44 |
| 14 | 50 | 35 | 50 | 2.16 |
| 15 | 50 | 35 | 50 | 2.6 |
| Factors | Centered and Scaled Coefficients | Coefficient Values | p |
|---|---|---|---|
| Constant | β0 | 2.627 | 0.000 * |
| Temperature (T) | β1 | 1.518 | 0.000 * |
| Ethanol (E) | β2 | 1.146 | 0.000 * |
| Amplitude (A) | β3 | 0.741 | 0.024 * |
| T × T | β11 | −0.326 | 0.382 |
| E × E | β22 | 0.834 | 0.016 * |
| A × A | β33 | 0.140 | 0.697 |
| T × E | β12 | 0.032 | 0.924 |
| T × A | β13 | −0.352 | 0.331 |
| E × A | β23 | 1.167 × 10−7 | 1 |
| Validation parameters for reduced model | |||
| R2 | 0.919 | ||
| R2 adj | 0.887 | ||
| K1 (min g DM/mg) | K2 (g DM/mg) | R2 | RMSE | R0 (mg/g DM min) | Ye (mg/g DM) | |
|---|---|---|---|---|---|---|
| Untreated | 0.124 ± 0.032 | 0.180 ± 0.000 | 0.998 | 0.082 | 8.638 ± 2.212 | 5.565 ± 0.014 |
| US | 0.120 ± 0.019 | 0.181 ± 0.005 | 0.996 | 0.139 | 7.784 ± 0.549 | 5.531 ± 0.074 |
| SC-CO2 | 0.095 ± 0.004 | 0.151 ± 0.001 | 0.998 | 0.108 | 10.517 ± 0.489 | 6.636 ± 0.037 |
| SC-CO2 + US | 0.057 ± 0.002 | 0.139 ± 0.000 | 0.997 | 0.136 | 17.697 ± 0.472 | 7.194 ± 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chadni, M.; Boussetta, N.; Guerin, C.; Lagalle, F.; Zoghlami, A.; Perré, P.; Allais, F.; Grimi, N.; Ioannou, I. Improvement of Sinapine Extraction from Mustard Seed Meal by Application of Emerging Technologies. Foods 2023, 12, 520. https://doi.org/10.3390/foods12030520
Chadni M, Boussetta N, Guerin C, Lagalle F, Zoghlami A, Perré P, Allais F, Grimi N, Ioannou I. Improvement of Sinapine Extraction from Mustard Seed Meal by Application of Emerging Technologies. Foods. 2023; 12(3):520. https://doi.org/10.3390/foods12030520
Chicago/Turabian StyleChadni, Morad, Nadia Boussetta, Cédric Guerin, Fabien Lagalle, Aya Zoghlami, Patrick Perré, Florent Allais, Nabil Grimi, and Irina Ioannou. 2023. "Improvement of Sinapine Extraction from Mustard Seed Meal by Application of Emerging Technologies" Foods 12, no. 3: 520. https://doi.org/10.3390/foods12030520