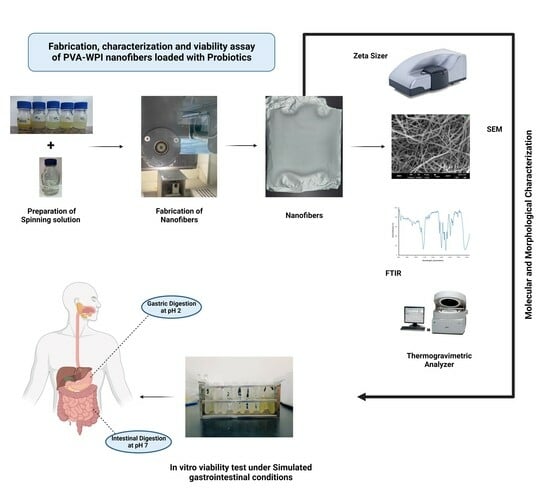
Fabrication and Characterization of PVA–WPI Based Nanofiber Mats for Improved Viability of Lactobacillus rhamnosus GG
Abstract
:1. Introduction
2. Materials and Methods
2.1. Procurement of the Material
2.2. Fabrication of Nanofiber Mats
2.2.1. Activation of Bacterial Culture
2.2.2. Preparation of Solutions
2.2.3. Characterization of Biopolymer Solution
2.2.4. Fabrication of Nanofiber Mats
2.3. Encapsulation Efficiency
2.4. Determination of Thickness and Mechnaical Properties of Nanofibers
2.5. Characterization of Blank and Probiotic-Loaded Nanofibers
2.5.1. Zeta Potential (ζ)
2.5.2. Scanning Electron Microscopy (SEM)
2.5.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.4. Thermogravimetric Analysis (TGA)
2.6. In Vitro Viability Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Properties of Biopolymer Solution
3.2. Encapsulation Efficiency
3.3. Mechanical Properties and Thickness of Nanofibers
3.4. Morphological and Molecular Characterization of Nanofiber Mats
3.4.1. Zeta Potential
3.4.2. Scanning Electron Microscopy (SEM)
3.4.3. FTIR Spectroscopy
3.4.4. Thermal Stability Analysis
3.5. Viability of Encapsulated and Free Cells under Simulated Gastrointestinal Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Şanlibaba, P.; Toprak, Z.T.; Tezel, B.U. The role of probiotics on slowing down the aging process. Acta Sci. Pol. Technol. Aliment. 2022, 21, 53–66. [Google Scholar] [PubMed]
- Reuben, R.C.; Elghandour, M.M.; Alqaisi, O.; Cone, J.W.; Márquez, O.; Salem, A.Z. Influence of microbial probiotics on ruminant health and nutrition: Sources, mode of action and implications. J. Sci. Food Agric. 2022, 102, 1319–1340. [Google Scholar] [CrossRef] [PubMed]
- Mörkl, S.; Butler, M.I.; Holl, A.; Cryan, J.F.; Dinan, T.G. Probiotics and the microbiota-gut-brain axis: Focus on psychiatry. Curr. Nutr. Rep. 2020, 9, 171–182. [Google Scholar] [CrossRef]
- Naik, S.S.; Anusha, G.; Leela, K.V.; Ravi, S. Promising Approaches in Drug Delivery Against Resistant Bacteria. In Advances in Novel Formulations for Drug Delivery; Wiley: Hoboken, NJ, USA, 2023; pp. 219–229. [Google Scholar]
- Simonič, M.; Slapničar, Š.; Trček, J.; Matijašić, B.B.; Lorbeg, P.M.; Vesel, A.; Zemljič, L.F.; Peršin Fratnik, Z. Probiotic Lactobacillus paragasseri K7 nanofiber encapsulation using nozzle-free electrospinning. Appl. Biochem. Biotechnol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Khan, M.A.; Chen, K.; Zhang, L.; Chen, X. Electrospinning of natural biopolymers for innovative food applications: A review. Food Bioprocess Technol. 2023, 16, 704–725. [Google Scholar] [CrossRef]
- Angel, N.; Li, S.; Yan, F.; Kong, L. Recent advances in electrospinning of nanofibers from bio-based carbohydrate polymers and their applications. Trends Food Sci. Technol. 2022, 120, 308–324. [Google Scholar] [CrossRef]
- Keramat, M.; Esteghlal, S.; Safari, J.; Golmakani, M.-T.; Khalesi, M. Fabrication of electrospun persian Gum/Poly (Vinyl Alcohol) and whey protein Isolate/Poly (vinyl alcohol) nanofibers incorporated with Oliveria decumbens Vent. essential oil. Nanosci. Nanotechnol. Asia 2019, 9, 371–380. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Shahamirian, M.; John, D.; Ramaswamy, H. Development and evaluation of antibacterial electrospun pea protein isolate-polyvinyl alcohol nanocomposite mats incorporated with cinnamaldehyde. Mater. Sci. Eng. C 2019, 94, 393–402. [Google Scholar] [CrossRef]
- Cho, D.; Netravali, A.N.; Joo, Y.L. Mechanical properties and biodegradability of electrospun soy protein Isolate/PVA hybrid nanofibers. Polym. Degrad. Stab. 2012, 97, 747–754. [Google Scholar] [CrossRef]
- Panahi, Z.; Mohsenzadeh, M.; Hashemi, M. Fabrication and characterization of PVA/WPI nanofibers containing probiotics using electrospinning technique. Nanomed. J. 2023, 10, 216–226. [Google Scholar]
- Aider-Kaci, F.A.; Aidarbekova, S.; Aider, M. Impact of electro-activated whey on growth, acid and bile resistance of Lacticaseibacillus rhamnosus GG and Lactobacillus acidophilus ATCC 4356. Heliyon 2023, 9, e13154. [Google Scholar] [CrossRef] [PubMed]
- Fareed, F.; Saeed, F.; Afzaal, M.; Imran, A.; Ahmad, A.; Mahmood, K.; Shah, Y.A.; Hussain, M.; Ateeq, H. Fabrication of electrospun gum Arabic–polyvinyl alcohol blend nanofibers for improved viability of the probiotic. J. Food Sci. Technol. 2022, 59, 4812–4821. [Google Scholar] [CrossRef] [PubMed]
- Çanga, E.M.; Dudak, F.C. Improved digestive stability of probiotics encapsulated within poly (vinyl alcohol)/cellulose acetate hybrid fibers. Carbohydr. Polym. 2021, 264, 117990. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, S.; Maryam, A. Encapsulation of lactic acid bacteria and Bifidobacteria using starch-sodium alginate nanofibers to enhance viability in food model. J. Food Process. Preserv. 2021, 45, e16048. [Google Scholar] [CrossRef]
- Duman, D.; Karadag, A. Inulin added electrospun composite nanofibres by electrospinning for the encapsulation of probiotics: Characterisation and assessment of viability during storage and simulated gastrointestinal digestion. Int. J. Food Sci. Technol. 2021, 56, 927–935. [Google Scholar] [CrossRef]
- Quezada-Pinedo, H.G.; Cassel, F.; Duijts, L.; Muckenthaler, M.U.; Gassmann, M.; Jaddoe, V.W.; Reiss, I.K.; Vermeulen, M.J. Maternal iron status in pregnancy and child health outcomes after birth: A systematic review and meta-analysis. Nutrients 2021, 13, 2221. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic acid: The influence of molecular weight on structural, physical, physico-chemical, and degradable properties of biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Agarwal, S.; Burgard, M.; Greiner, A.; Wendorff, J. Electrospinning: A Practical Guide to Nanofibers; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2016. [Google Scholar]
- Suresh, S.; Becker, A.; Glasmacher, B. Impact of apparatus orientation and gravity in electrospinning—A review of empirical evidence. Polymers 2020, 12, 2448. [Google Scholar] [CrossRef]
- Ardestani, S.A.; Ghanbarzadeh, B.; Moini, S. The improvement of the sodium caseinate based electrospun nanofiber by modifying solvent system: Study of microstructure and physical properties. Food Hydrocoll. 2023, 137, 108747. [Google Scholar] [CrossRef]
- Adeli, H.; Khorasani, M.T.; Parvazinia, M. Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: Fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int. J. Biol. Macromol. 2019, 122, 238–254. [Google Scholar] [CrossRef]
- Sanderson, H.; Khan, K.; Brun Hansen, A.M.; Connors, K.; Lam, M.W.; Roy, K.; Belanger, S. Environmental toxicity (Q) SARs for polymers as an emerging class of materials in regulatory frameworks, with a focus on challenges and possibilities regarding cationic polymers. In Ecotoxicological QSARs; Humana: New York, NY, USA, 2020; pp. 681–705. [Google Scholar]
- Zhang, X.; Wang, R.; Cheng, C.; Zhang, Y.; Ma, Y.; Lu, W. Identification of two novel dipeptidyl peptidase-IV inhibitory peptides from sheep whey protein and inhibition mechanism revealed by molecular docking. Food Biosci. 2022, 48, 101733. [Google Scholar] [CrossRef]
- Ilango, P.R.; Savariraj, A.D.; Huang, H.; Li, L.; Hu, G.; Wang, H.; Hou, X.; Kim, B.C.; Ramakrishna, S.; Peng, S. Electrospun Flexible Nanofibres for Batteries: Design and Application. Electrochem. Energy Rev. 2023, 6, 12. [Google Scholar]
- Ma, J.; Li, T.; Wang, Q.; Xu, C.; Yu, W.; Yu, H.; Wang, W.; Feng, Z.; Chen, L.; Hou, J. Enhanced viability of probiotics encapsulated within synthetic/natural biopolymers by the addition of gum arabic via electrohydrodynamic processing. Food Chem. 2023, 413, 135680. [Google Scholar] [CrossRef] [PubMed]
- Centurion, F.; Basit, A.W.; Liu, J.; Gaisford, S.; Rahim, M.A.; Kalantar-Zadeh, K. Nanoencapsulation for probiotic delivery. ACS Nano 2021, 15, 18653–18660. [Google Scholar] [CrossRef] [PubMed]
- Smruthi, M.; Nallamuthu, I.; Anand, T. A comparative study of optimized naringenin nanoformulations using nano-carriers (PLA/PVA and zein/pectin) for improvement of bioavailability. Food Chem. 2022, 369, 130950. [Google Scholar] [CrossRef]
- Nie, A.; Bu, Y.; Li, P.; Zhang, Y.; Jin, T.; Liu, J.; Su, Z.; Wang, Y.; He, J.; Liu, Z. Approaching diamond’s theoretical elasticity and strength limits. Nat. Commun. 2019, 10, 5533. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Li, T.; Xiao, P. Study on the characteristics of elongation at break and tensile strength of photovoltaic insulating backsheets subjected to partial discharge degradation. AIP Adv. 2021, 11, 045305. [Google Scholar] [CrossRef]
- Wu, S.; Peng, S.; Yu, Y.; Wang, C.H. Strategies for designing stretchable strain sensors and conductors. Adv. Mater. Technol. 2020, 5, 1900908. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Ahmadigol, A.; Khubber, S.; Altintas, Z. Bionanocomposite films with plasticized WPI-jujube polysaccharide/starch nanocrystal blends for packaging fresh-cut carrots. Food Packag. Shelf Life 2023, 36, 101042. [Google Scholar] [CrossRef]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Sapuan, S.; Ilyas, R. Highly transparent and antimicrobial PVA based bionanocomposites reinforced by ginger nanofiber. Polym. Test. 2020, 81, 106186. [Google Scholar] [CrossRef]
- Ali, M.A.S.S.; Jimat, D.N.; Nawawi, W.M.F.W.; Sulaiman, S. Antibacterial, mechanical and thermal properties of PVA/starch composite film reinforced with cellulose nanofiber of sugarcane bagasse. Arab. J. Sci. Eng. 2022, 47, 5747–5754. [Google Scholar] [CrossRef]
- Matter, F.; Luna, A.L.; Niederberger, M. From colloidal dispersions to aerogels: How to master nanoparticle gelation. Nano Today 2020, 30, 100827. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Taylan, O.; Karakas, C.Y.; Dertli, E. An alternative way to encapsulate probiotics within electrospun alginate nanofibers as monitored under simulated gastrointestinal conditions and in kefir. Carbohydr. Polym. 2020, 244, 116447. [Google Scholar] [CrossRef] [PubMed]
- Cacua, K.; Ordoñez, F.; Zapata, C.; Herrera, B.; Pabón, E.; Buitrago-Sierra, R. Surfactant concentration and pH effects on the zeta potential values of alumina nanofluids to inspect stability. Colloids Surf. A: Physicochem. Eng. Asp. 2019, 583, 123960. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Primavera, R.; Wilson, R.J.; Thakor, A.S.; Kevadiya, B.D. Cellular uptake and retention of nanoparticles: Insights on particle properties and interaction with cellular components. Mater. Today Commun. 2020, 25, 101692. [Google Scholar] [CrossRef]
- Rajati, H.; Alvandi, H.; Rahmatabadi, S.S.; Hosseinzadeh, L.; Arkan, E. A nanofiber-hydrogel composite from green synthesized AgNPs embedded to PEBAX/PVA hydrogel and PA/Pistacia atlantica gum nanofiber for wound dressing. Int. J. Biol. Macromol. 2023, 226, 1426–1443. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, Z. Use of characterized chitosan nanoparticles integrated in poly (vinyl alcohol) nanofibers as an alternative nanoscale material for fish balls. J. Food Saf. 2018, 38, e12551. [Google Scholar] [CrossRef]
- Ong, H.C.; Chen, W.-H.; Singh, Y.; Gan, Y.Y.; Chen, C.-Y.; Show, P.L. A state-of-the-art review on thermochemical conversion of biomass for biofuel production: A TG-FTIR approach. Energy Convers. Manag. 2020, 209, 112634. [Google Scholar] [CrossRef]
- Kharazmi, A.; Faraji, N.; Hussin, R.M.; Saion, E.; Yunus, W.M.M.; Behzad, K. Structural, optical, opto-thermal and thermal properties of ZnS–PVA nanofluids synthesized through a radiolytic approach. Beilstein J. Nanotechnol. 2015, 6, 529–536. [Google Scholar] [CrossRef]
- Gbassi, G.; Yolou, F.; Sarr, S.; Atheba, P.; Amin, C.; Ake, M. Whey proteins analysis in aqueous medium and in artificial gastric and intestinal fluids. Int. J. Biol. Chem. Sci. 2012, 6, 1828–1837. [Google Scholar] [CrossRef]
- Warji, W.; Purwanti, N.; Mardjan, S.; Yuliani, S. Temperature and Heating Time of Forming Process of Nanofibrils of Whey Protein Isolate. In Proceedings of the IOP Conference Series: Earth and Environmental Science, South Lampung, Indonesia, 23–25 October 2020; p. 012067. [Google Scholar]
- Roberts, D.; Reyes, V.; Bonilla, F.; Dzandu, B.; Liu, C.; Chouljenko, A.; Sathivel, S. Viability of Lactobacillus plantarum NCIMB 8826 in fermented apple juice under simulated gastric and intestinal conditions. LWT 2018, 97, 144–150. [Google Scholar] [CrossRef]
- Feng, K.; Huang, R.-M.; Wu, R.-Q.; Wei, Y.-S.; Zong, M.-H.; Linhardt, R.J.; Wu, H. A novel route for double-layered encapsulation of probiotics with improved viability under adverse conditions. Food Chem. 2020, 310, 125977. [Google Scholar] [CrossRef] [PubMed]




| Mat Blends | Ratios of Blends | Concentration in Grams | ||
|---|---|---|---|---|
| 10% PVA | 3% WPI | 10% PVA | 3% WPI | |
| M0 | 100 | 0 | 40 mL | 0 mL |
| M1 | 95 | 5 | 38 mL | 2 mL |
| M2 | 90 | 10 | 36 mL | 4 mL |
| M3 | 85 | 15 | 34 mL | 6 mL |
| M4 | 80 | 20 | 32 mL | 8 mL |
| M5 | 75 | 25 | 30 mL | 10 mL |
| Parameter | M0 | M1 | M2 | M3 | M4 | M5 |
|---|---|---|---|---|---|---|
| Conductivity (milli-Siemens/cm) | 1.62 ± 0.01 | 3.33 ± 0.005 | 3.52 ± 0.001 | 3.71 ± 0.002 | 3.82 ± 0.006 | 4.92 ± 0.004 |
| Viscosity (mPa·s) | 1213.1 ± 0.05 | 708.47 ± 0.05 | 533.44 ± 0.06 | 358.88 ± 0.05 | 186.85 ± 0.04 | 84.096 ± 0.01 |
| Surface tension (nM/m) | 49.44 ± 0.07 | 34.23 ± 0.04 | 29.94 ± 0.05 | 25.82 ± 0.08 | 21.66 ± 0.04 | 19.55 ± 0.05 |
| Treatment | M1 | M2 | M3 | M4 | M5 |
|---|---|---|---|---|---|
| Encapsulation efficiency % | 81.67 | 83.55 | 85.55 | 89.37 | 93.75 |
| Parameter | M0 | M1 | M2 | M3 | M4 | M5 |
|---|---|---|---|---|---|---|
| Zeta potential (mV) | −7.66 ± 0.08 | −8.63 ± 0.01 | −8.88 ± 0.02 | 9.12 ± 0.03 | −9.42 ± 0.04 | −9.64 ± 0.03 |
| Barrier Properties | ||||||
| Thickness (mm) | 0.12 ± 0.01 | 0.14 ± 0.02 | 0.15 ± 0.02 | 0.16 ± 0.01 | 0.17 ± 0.01 | 0.18 ± 0.01 |
| Tensile strength (MPa) | 13.36 ± 0.04 | 14.25 ± 0.04 | 15.12 ± 0.02 | 16.05 ± 0.01 | 15.58 ± 0.02 | 17.82 ± 0.01 |
| Elongation at break (%) | 18.88 ± 0.04 | 26.81 ± 0.01 | 28.11 ± 0.01 | 29.11 ± 0.02 | 30.90 ± 0.01 | 32.70 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akram, N.; Afzaal, M.; Saeed, F.; Ahmad, A.; Imran, A.; Ahmed, A.; Shah, Y.A.; Islam, F.; Alomar, S.Y.; Manoharadas, S.; et al. Fabrication and Characterization of PVA–WPI Based Nanofiber Mats for Improved Viability of Lactobacillus rhamnosus GG. Foods 2023, 12, 3904. https://doi.org/10.3390/foods12213904
Akram N, Afzaal M, Saeed F, Ahmad A, Imran A, Ahmed A, Shah YA, Islam F, Alomar SY, Manoharadas S, et al. Fabrication and Characterization of PVA–WPI Based Nanofiber Mats for Improved Viability of Lactobacillus rhamnosus GG. Foods. 2023; 12(21):3904. https://doi.org/10.3390/foods12213904
Chicago/Turabian StyleAkram, Noor, Muhammad Afzaal, Farhan Saeed, Adnan Ahmad, Ali Imran, Aftab Ahmed, Yasir Abbas Shah, Fakhar Islam, Suliman Yousef Alomar, Salim Manoharadas, and et al. 2023. "Fabrication and Characterization of PVA–WPI Based Nanofiber Mats for Improved Viability of Lactobacillus rhamnosus GG" Foods 12, no. 21: 3904. https://doi.org/10.3390/foods12213904