Processing Stage-Induced Formation of Advanced Glycation End Products in Cooked Sausages with the Addition of Spices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
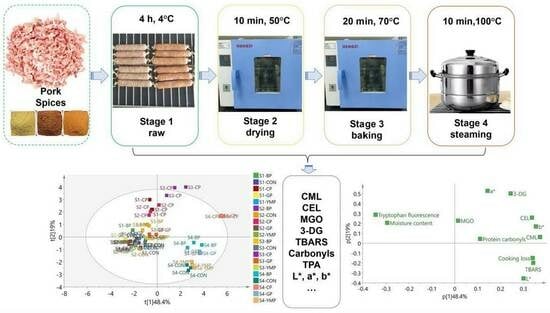
2.2. Preparation of Sausages
2.3. Physicochemical Analysis
2.3.1. Determination of Cooking Loss and Moisture Content
2.3.2. Texture Profile Analysis (TPA)
2.3.3. Color
2.4. Lipid and Protein Oxidation Indicators
2.4.1. Lipid Oxidation
2.4.2. Tryptophan Fluorescence
2.4.3. Protein Carbonyls
2.5. Measurement of AGEs
2.6. Measurement of 1,2-Dicarbonyl Compounds
2.7. Statistical Analyses
3. Results and Discussion
3.1. Physicochemical Analysis
3.1.1. Changes in Cooking Loss and Moisture Content
3.1.2. Changes in Color Parameters and TPA
3.2. Lipids and Protein Oxidation in Sausages during the Different Processing Stages
3.2.1. Lipid Oxidation
3.2.2. Changes in Tryptophan Fluorescence Intensity
3.2.3. Changes in Protein Carbonyls
3.3. Changes in AGE Profiles
3.4. Changes in 1,2-Dicarbonyl Compounds
3.5. Principal Component Analysis (PCA) and Correlation Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Huang, M.; Cheng, Y.; Khan, I.A.; Huang, J. A comprehensive review of Nε-carboxymethyllysine and Nε-carboxyethyllysine in thermal processed meat products. Trends Food Sci. 2020, 98, 30–40. [Google Scholar] [CrossRef]
- Jia, W.; Guo, A.; Zhang, R.; Shi, L. Mechanism of natural antioxidants regulating advanced glycosylation end products of Maillard reaction. Food Chem. 2023, 404, 134541. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Sun, X.; Tang, J.; Wang, J.; Wang, J.; Rasco, B.A.; Lai, K.; Fan, Y.; Huang, Y. Combination effects of salts and cold storage on the formation of protein-bound Nε-(carboxymethyl) lysine and Nε-(carboxyethyl) lysine in raw and subsequently commercially sterilized ground pork. Food Chem. 2018, 264, 455–461. [Google Scholar] [CrossRef]
- Sun, X.; Li, X.; Tang, J.; Lai, K.; Rasco, B.A.; Huang, Y. Formation of protein-bound Nε-carboxymethyllysine and Nε-carboxyethyllysine in ground pork during commercial sterilization as affected by the type and concentration of sugars. Food Chem. 2021, 336, 127706. [Google Scholar] [CrossRef]
- Shen, Z.; Li, S.; Wu, J.; Wang, F.; Li, X.; Yu, J.; Liu, Y.; Ma, X. Effect of different oil incorporation on gelling properties, flavor and advanced glycation end-products of silver carp surimi sausages. J. Food Meas. Charact. 2022, 16, 5007–5022. [Google Scholar] [CrossRef]
- Uribarri, J.; del Castillo, M.D.; de la Maza, M.P.; Filip, R.; Gugliucci, A.; Luevano-Contreras, C.; Macías-Cervantes, M.H.; Markowicz Bastos, D.H.; Medrano, A.; Menini, T. Dietary advanced glycation end products and their role in health and disease. Adv. Nutr. 2015, 6, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Snelson, M.; Coughlan, M.T. Dietary advanced glycation end products: Digestion, metabolism and modulation of gut microbial ecology. Nutrients 2019, 11, 215. [Google Scholar] [CrossRef]
- Lu, J.; Li, M.; Shen, M.; Xie, J.; Xie, M. Advanced glycation end products and nitrosamines in sausages influenced by processing parameters, food additives and fat during thermal processing. Foods 2023, 12, 394. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, Y.; Gao, C.; Yang, Y.; Zeng, M.; Chen, J. N(ε)-carboxymethyl-lysine and N(ε)-carboxyethyl-lysine contents in commercial meat products. Food Res. Int. 2022, 155, 111048. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Soladoye, O.; Juárez, M.; Aalhus, J.; Shand, P.; Estévez, M. Protein oxidation in processed meat: Mechanisms and potential implications on human health. Compr. Rev. Food Sci. Food Saf. 2015, 14, 106–122. [Google Scholar] [CrossRef]
- Liu, Z.; Xiong, Y.L. Chen, J. Morphological examinations of oxidatively stressed pork muscle and myofibrils upon salt marination and cooking to elucidate the water-binding potential. J. Agric. Food Chem. 2011, 59, 13026–13034. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Fang, R.; Huang, M.; Wei, Y.; Zhou, G. Oxidation combined with Maillard reaction induced free and protein-bound Nε-carboxymethyllysine and Nε-carboxyethyllysine formation during braised chicken processing. Food Sci. Hum. Wellness 2020, 9, 383–393. [Google Scholar] [CrossRef]
- Starowicz, M.; Zieliński, H. Inhibition of advanced glycation end-product formation by high antioxidant-leveled spices commonly used in European cuisine. Antioxidants 2019, 8, 100. [Google Scholar] [CrossRef]
- Yang, D.; He, Z.; Wang, Z.; Fang, Q.; Oz, F.; Chen, J.; Zeng, M. Processing stage-guided effects of spices on the formation and accumulation of heterocyclic amines in smoked and cooked sausages. Food Biosci. 2022, 47, 101776. [Google Scholar] [CrossRef]
- Karre, L.; Lopez, K.; Getty, K.J. Natural antioxidants in meat and poultry products. Meat Sci. 2013, 94, 220–227. [Google Scholar] [CrossRef]
- Kim, H.-W.; Choi, J.-H.; Choi, Y.-S.; Kim, H.-Y.; Lee, M.-A.; Hwang, K.-E.; Song, D.-H.; Lee, J.-W.; Kim, C.-J. Effects of kimchi and smoking on quality characteristics and shelf life of cooked sausages prepared with irradiated pork. Meat Sci. 2014, 96, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Utrera, M.; Estevez, M. Oxidation of myofibrillar proteins and impaired functionality: Underlying mechanisms of the carbonylation pathway. J. Agric. Food Chem. 2012, 60, 8002–8011. [Google Scholar] [CrossRef]
- Gan, X.; Li, H.; Wang, Z.; Emara, A.; Zhang, D.; He, Z. Does protein oxidation affect proteolysis in low sodium Chinese traditional bacon processing? Meat Sci. 2019, 150, 14–22. [Google Scholar] [CrossRef]
- Cando, D.; Morcuende, D.; Utrera, M.; Estévez, M. Phenolic-rich extracts from Willowherb (Epilobium hirsutum L.) inhibit lipid oxidation but accelerate protein carbonylation and discoloration of beef patties. Eur. Food Res. Technol. 2014, 238, 741–751. [Google Scholar] [CrossRef]
- Li, Y.; Xue, C.; Quan, W.; Qin, F.; Wang, Z.; He, Z.; Zeng, M.; Chen, J. Assessment the influence of salt and polyphosphate on protein oxidation and Nε-(carboxymethyl) lysine and Nε-(carboxyethyl) lysine formation in roasted beef patties. Meat Sci. 2021, 177, 108489. [Google Scholar] [CrossRef]
- Bao, X.; Miao, J.; Fan, Y.; Lai, K. The effective inhibition of the formation of heterocyclic aromatic amines via adding black pepper in fried tilapia fillets. J. Food Process. Preserv. 2020, 44, e14435. [Google Scholar] [CrossRef]
- Fernandes, R.d.P.P.; Trindade, M.A.; Lorenzo, J.; De Melo, M. Assessment of the stability of sheep sausages with the addition of different concentrations of Origanum vulgare extract during storage. Meat Sci. 2018, 137, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, T.; Ho, P.; Goethals, S.; De Smet, S. The potential of herbs and spices to reduce lipid oxidation during heating and gastrointestinal digestion of a beef product. Food Res. Int. 2017, 102, 785–792. [Google Scholar] [CrossRef]
- Aguirrezábal, M.M.; Mateo, J.; Domínguez, M.C.; Zumalacárregui, J.M. The effect of paprika, garlic and salt on rancidity in dry sausages. Meat Sci. 2000, 54, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.-S.S.; Shehata, M.G.; Abd-Rabou, H.S.; El-Menshawy, H. Extend shelf-life of vacuum-packaged herring fish fillets using garlic and ginger extracts. J. Pure Appl. Microbiol. 2019, 13, 1571–1581. [Google Scholar] [CrossRef]
- Puvača, N.; Kostadinović, L.; Popović, S.; Lević, J.; Ljubojević, D.; Tufarelli, V.; Jovanović, R.; Tasić, T.; Ikonić, P.; Lukač, D. Proximate composition, cholesterol concentration and lipid oxidation of meat from chickens fed dietary spice addition (Allium sativum, Piper nigrum, Capsicum annuum). Anim. Prod. Sci. 2015, 56, 1920–1927. [Google Scholar] [CrossRef]
- Sun, W.; Cui, C.; Zhao, M.; Zhao, Q.; Yang, B. Effects of composition and oxidation of proteins on their solubility, aggregation and proteolytic susceptibility during processing of Cantonese sausage. Food Chem. 2011, 124, 336–341. [Google Scholar] [CrossRef]
- Roldan, M.; Antequera, T.; Armenteros, M.; Ruiz, J. Effect of different temperature–time combinations on lipid and protein oxidation of sous-vide cooked lamb loins. Food Chem. 2014, 149, 129–136. [Google Scholar] [CrossRef]
- Sun, X.; Tang, J.; Wang, J.; Rasco, B.A.; Lai, K.; Huang, Y. Formation of free and protein-bound carboxymethyllysine and carboxyethyllysine in meats during commercial sterilization. Meat Sci. 2016, 116, 1–7. [Google Scholar] [CrossRef]
- Wang, W.; Kou, Y.; Du, Y.; Li, M.; Zhang, J.; Yan, A.; Xie, J.; Shen, M. Investigation on the contents of Nε-carboxymethyllysine, Nε-carboxyethyllysine, and N-nitrosamines in commercial sausages on the Chinese market. Foods 2023, 12, 724. [Google Scholar] [CrossRef]
- Bai, X.; Li, Y.; Liang, W.; Xia, X.; Bian, C. Formation of advanced glycation end products of chicken breast meat induced by freeze-thaw cycles and subsequent cooking. Int. J. Biol. Macromol. 2023, 244, 125387. [Google Scholar] [CrossRef]
- Yu, L.; Gao, C.; Zeng, M.; He, Z.; Wang, L.; Zhang, S.; Chen, J. Effects of raw meat and process procedure on Nε-carboxymethyllysine and Nε-carboxyethyl-lysine formation in meat products. Food Sci. Biotechnol. 2016, 25, 1163–1168. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Huang, S.F.; Liao, K.W.; Ho, C.T.; Hung, W.L. Quantitation of α-dicarbonyls, lysine-and arginine-derived advanced glycation end products, in commercial canned meat and seafood products. J. Agric. Food Chem. 2023, 71, 6727–6737. [Google Scholar] [CrossRef] [PubMed]
- Yusufoğlu, B.; Yaman, M.; Karakuş, E. Determination of the most potent precursors of advanced glycation end products in some high-sugar containing traditional foods using high-performance liquid chromatography. J. Food Process. Preserv. 2020, 44, e14708. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Y.; Xue, C.; He, Z.; Wang, Z.; Qin, F.; Chen, J.; Zeng, M. The inhibitory effects of yellow mustard (Brassica juncea) and its characteristic pungent ingredient allyl isothiocyanate (AITC) on PhIP formation: Focused on the inhibitory pathways of AITC. Food Chem. 2022, 373, 131398. [Google Scholar] [CrossRef]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47 (Suppl. S1), 3–27. [Google Scholar] [CrossRef] [PubMed]
- Jono, T.; Nagai, R.; Lin, X.; Ahmed, N.; Thornalley, P.J.; Takeya, M.; Horiuchi, S. Nepsilon-(Carboxymethyl)lysine and 3-DG-imidazolone are major AGE structures in protein modification by 3-deoxyglucosone. J. Biochem. 2004, 136, 351–358. [Google Scholar] [CrossRef]


| Cooking Loss (%) | Moisture Content (%) | Color Parameters | ||||
|---|---|---|---|---|---|---|
| L* (Lightness) | a* (Redness) | b* (Yellowness) | ||||
| Control | raw | _ | 52.78 ± 0.26 aC | 45.15 ± 4.68 bA | 2.41 ± 0.50 aB | 12.17 ± 1.19 bB |
| drying | 1.23 ± 0.27 aB | 49.43 ± 0.13 bD | 42.14 ± 3.33 bcA | 2.01 ± 0.51 aB | 10.69 ± 1.69 bB | |
| baking | 4.75 ± 0.84 bB | 45.73 ± 0.10 cD | 39.19 ± 2.70 cA | 1.26 ± 0.43 bB | 10.41 ± 1.63 bBC | |
| steaming | 9.84 ± 2.07 cB | 42.67 ± 0.11 dE | 57.50 ± 2.06 aA | 1.30 ± 0.36 bD | 15.27 ± 1.75 aD | |
| Black pepper | raw | _ | 51.96 ± 0.32 aD | 40.59 ± 4.72 bB | 2.54 ± 0.80 aB | 10.48 ± 1.67 bB |
| drying | 1.37 ± 0.28 aB | 51.07 ± 0.26 bB | 41.04 ± 3.97 bA | 1.29 ± 0.36 bC | 7.90 ± 0.73 cC | |
| baking | 5.46 ± 0.72 bB | 49.20 ± 0.17 cA | 37.60 ± 2.86 bA | 1.61 ± 0.3 bB | 9.45 ± 1.54 bC | |
| steaming | 12.76 ± 2.56 cB | 47.91 ± 0.16 dA | 51.47 ± 1.92 aB | 2.56 ± 0.28 aB | 16.99 ± 0.80 aC | |
| Chili | raw | _ | 53.36 ± 0.24 aB | 41.90 ± 1.93 bAB | 9.16 ± 1.38 aA | 17.94 ± 2.16 bA |
| drying | 1.45 ± 0.29 aB | 51.73 ± 0.19 bA | 42.54 ± 3.51 bA | 6.81 ± 0.77 bA | 15.16 ± 2.60 cA | |
| baking | 5.36 ± 0.42 bB | 47.58 ± 0.12 cC | 37.97 ± 2.84 cA | 7.08 ± 0.87 bA | 16.70 ± 1.81 bcA | |
| steaming | 20.88 ± 2.47 cA | 46.95 ± 0.14 dB | 52.03 ± 1.27 aB | 7.35 ± 0.56 bA | 23.87 ± 1.05 aA | |
| Yellow mustard | raw | _ | 53.16 ± 0.21 aBC | 44.03 ± 2.47 bAB | 2.96 ± 1.19 aB | 12.32 ± 2.14 bB |
| drying | 1.33 ± 0.33 aB | 50.94 ± 0.23 bB | 43.02 ± 2.93 bA | 1.25 ± 0.43 bCD | 10.95 ± 1.31 bcB | |
| baking | 4.78 ± 0.91 bB | 49.26 ± 0.10 cA | 39.95 ± 2.39 cA | 1.41 ± 0.36 bB | 9.89 ± 1.47 cC | |
| steaming | 19.53 ± 3.15 cA | 45.4 ± 0.14 dD | 56.73 ± 1.56 aA | 1.96 ± 0.29 bC | 18.11 ± 1.13 aBC | |
| Garlic | raw | _ | 55.29 ± 0.21 aA | 42.06 ± 3.57 bAB | 0.69 ± 0.24 bC | 11.48 ± 1.85 bB |
| drying | 1.82 ± 0.38 aA | 50.06 ± 0.32 bC | 40.59 ± 2.34 bA | 0.73 ± 0.14 bD | 10.37 ± 1.23 bB | |
| baking | 6.35 ± 0.45 bA | 48.78 ± 0.17 cB | 39.76 ± 3.03 bA | 0.70 ± 0.20 bC | 11.79 ± 0.72 bB | |
| steaming | 21.36 ± 1.87 cA | 45.93 ± 0.15 dC | 51.90 ± 1.79 aB | 2.33 ± 0.39 aBC | 18.85 ± 1.01 aB | |
| Steamed | Hardness | Adhesiveness | Cohesiveness | Springiness | Gumminess | Chewiness |
|---|---|---|---|---|---|---|
| Sausage | N | N.mm | Ratio | mm | N | mJ |
| Control | 31.4 ± 2.98 BC | 0.11 ± 0.02 A | 0.22 ± 0.01 A | 9.4 ± 0.5 AB | 6.8 ± 0.57 BC | 64.05 ± 7.54 AB |
| Black pepper | 34.32 ± 3.2 AB | 0.1 ± 0.02 A | 0.21 ± 0.03 A | 8.95 ± 1.31 B | 7.36 ± 1.31 ABC | 70.09 ± 15.77 AB |
| Chili | 34.67 ± 2.64 A | 0.1 ± 0.02 A | 0.22 ± 0.02 A | 9.62 ± 0.47 A | 7.66 ± 1.04 AB | 73.79 ± 11.93 A |
| Yellow mustard | 28.45 ± 1.99 C | 0.11 ± 0.03 A | 0.22 ± 0.02 A | 9.44 ± 0.22 AB | 6.23 ± 0.94 C | 58.9 ± 10.33 B |
| Garlic | 36.11 ± 2.67 A | 0.09 ± 0.02 A | 0.22 ± 0.02 A | 9.63 ± 0.49 A | 7.89 ± 0.87 A | 76.27 ± 11.85 A |
| Spices | Stages | TBARS (mg MDA/kg) | Tryptophan Fluorescence Intensity | Total Carbonyl Content (nmol/mg Protein) |
|---|---|---|---|---|
| Control | Raw | 0.28 ± 0.0062 bB | 993.00 ± 15.56 bB | 6.12 ± 0.34 aA |
| Drying | 0.24 ± 0.035 bAB | 1058.50 ± 31.82 aB | 6.40 ± 0.96 aA | |
| Baking | 0.25 ± 0.031 bD | 989.50 ± 16.26 bB | 6.29 ± 0.10 aAB | |
| Steaming | 2.11 ± 0.028 aE | 661.50 ± 4.95 cB | 6.45 ± 0.35 aB | |
| Black pepper | Raw | 0.27 ± 0.0078 cBC | 1086.00 ± 32.53 aA | 5.04 ± 2.04 cA |
| Drying | 0.25 ± 0.011 cA | 1061.50 ± 95.46 aB | 6.09 ± 1.93 bcA | |
| Baking | 1.71 ± 0.063 bC | 1196.50 ± 4.95 aA | 8.22 ± 1.25 abA | |
| Steaming | 2.85 ± 0.038 aD | 803.50 ± 2.12 bA | 9.37 ± 1.04 aA | |
| Chili | Raw | 0.26 ± 0.0085 bC | 1090.00 ± 62.23 aA | 5.78 ± 0.91 aA |
| Drying | 0.23 ± 0.0077 cAB | 1118.00 ± 26.87 aAB | 6.52 ± 0.30 aA | |
| Baking | 0.28 ± 0.016 bD | 1075.00 ± 131.52 aAB | 5.57 ± 1.04 aB | |
| Steaming | 3.22 ± 0.033 aB | 731.00 ± 4.24 bAB | 6.71 ± 0.15 aB | |
| Yellow mustard | Raw | 0.27 ± 0.0094 cBC | 1063.50 ± 0.71 aAB | 4.65 ± 0.66 bcA |
| Drying | 0.21 ± 0.018 cB | 1112.50 ± 27.58 aAB | 6.19 ± 1.30 abA | |
| Baking | 2.29 ± 0.042 bA | 1096.50 ± 33.23 aAB | 7.49 ± 0.79 aA | |
| Steaming | 3.06 ± 0.11 aC | 703.50 ± 20.51 bAB | 4.80 ± 1.066 cC | |
| Garlic | Raw | 0.29 ± 0.0027 cA | 1087.00 ± 5.66 aA | 5.89 ± 0.79 aA |
| Drying | 0.22 ± 0.0085 dAB | 1232.50 ± 10.61 aA | 5.92 ± 2.17 aA | |
| Baking | 1.98 ± 0.069 bB | 1127.00 ± 111.72 aAB | 6.62 ± 0.77 aAB | |
| Steaming | 3.34 ± 0.059 aA | 710.5 ± 30.41 bAB | 7.85 ± 0.91 aB | |
| Spices | <0.001 | 0.324 | <0.001 | |
| Stages | <0.001 | 0.004 | <0.001 | |
| Spices * Stages | <0.001 | 0.003 | <0.001 |
| Spices | Stages | CML (μg/g) | CEL (μg/g) |
|---|---|---|---|
| Control | Raw | 4.32 ± 0.19 cA | 7.74 ± 1.56 bA |
| Drying | 5.90 ± 0.42 bBC | 6.66 ± 1.93 bB | |
| Baking | 6.49 ± 0.35 bB | 9.35 ± 1.70 bB | |
| Steaming | 12.69 ± 0.28 aBC | 13.61 ± 1.74 aC | |
| Black pepper | Raw | 4.26 ± 0.57 bA | 7.95 ± 1.76 bA |
| Drying | 4.93 ± 0.59 bBC | 5.38 ± 1.19 bB | |
| Baking | 6.89 ± 0.48 bB | 15.26 ± 4.23 aB | |
| Steaming | 16.22 ± 3.26 aA | 18.56 ± 3.87 aB | |
| Chili | Raw | 4.81 ± 1.42 cA | 7.34 ± 2.36 bAB |
| Drying | 8.15 ± 1.38 bA | 13.44 ± 3.47 bA | |
| Baking | 13.96 ± 1.35 aA | 25.25 ± 7.87 aA | |
| Steaming | 14.82 ± 1.17 aAB | 27.24 ± 2.25 aA | |
| Yellow mustard | Raw | 4.80 ± 0.22 cA | 4.79 ± 0.77 cB |
| Drying | 4.25 ± 0.91 cC | 5.92 ± 0.92 cB | |
| Baking | 6.39 ± 0.32 bB | 11.18 ± 1.32 bB | |
| Steaming | 10.68 ± 0.57 aC | 13.42 ± 0.56 aC | |
| Garlic | Raw | 4.42 ± 0.40 cA | 6.42 ± 0.94 bAB |
| Drying | 6.24 ± 1.37 bB | 7.11 ± 1.98 bB | |
| Baking | 7.22 ± 0.21 bB | 12.95 ± 3.88 aB | |
| Steaming | 11.07 ± 0.94 aC | 16.65 ± 2.17 aBC |
| CML | CEL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | SS | DF | Mean Square | F | Pr > F | SS | DF | Mean Square | F | Pr > F |
| Model a | 860.186 a | 19 | 45.273 | 38.696 | <0.001 | 2310.857 a | 19 | 121.624 | 15.020 | <0.001 |
| Stages b | 638.023 | 3 | 212.674 | 181.779 | <0.001 | 1311.770 | 3 | 437.257 | 54.000 | <0.001 |
| Spices c | 108.955 | 4 | 27.239 | 23.282 | <0.001 | 701.027 | 4 | 175.257 | 21.644 | <0.001 |
| Stages * Spices | 113.209 | 12 | 9.434 | 8.064 | <0.001 | 298.060 | 12 | 24.838 | 3.067 | 0.004 |
| Error | 46.798 | 40 | 1.170 | 323.893 | 40 | 8.097 | ||||
| Corrected total | 906.985 | 59 | 2634.750 | 59 | ||||||
| Spices | Stages | MGO (μg/g) | 3-DG (μg/g) |
|---|---|---|---|
| Control | Raw | 0.093 ± 0.013 bA | 0.020 ± 0.00034 cC |
| Drying | 0.091 ± 0.025 bA | 0.022 ± 0.0034 bcC | |
| Baking | 0.25 ± 0.031 aB | 0.030 ± 0.0035 aE | |
| Steaming | 0.11 ± 0.022 bA | 0.029 ± 0.0057 abE | |
| Black pepper | Raw | 0.065 ± 0.0055 cB | 0.046 ± 0.0099 cB |
| Drying | 0.071 ± 0.0053 cAB | 0.057 ± 0.0038 bcB | |
| Baking | 0.27 ± 0.017 aB | 0.070 ± 0.012 abC | |
| Steaming | 0.12 ± 0.0052 bA | 0.086 ± 0.0042 aC | |
| Chili | Raw | 0.080 ± 0.015 bAB | 0.17 ± 0.0080 cA |
| Drying | 0.069 ± 0.0070 bAB | 0.17 ± 0.015 cA | |
| Baking | 0.33 ± 0.055 aA | 0.25 ± 0.0072 bA | |
| Steaming | 0.073 ± 0.011 bB | 0.29 ± 0.014 aA | |
| Yellow mustard | Raw | 0.085 ± 0.0023 bA | 0.026 ± 0.0096 bC |
| Drying | 0.082 ± 0.010 bAB | 0.028 ± 0.0020 bC | |
| Baking | 0.28 ± 0.0073 aB | 0.051 ± 0.0058 aD | |
| Steaming | 0.089 ± 0.0085 bB | 0.056 ± 0.0018 aD | |
| Garlic | Raw | 0.078 ± 0.0051 bAB | 0.044 ± 0.0055 cB |
| Drying | 0.065 ± 0.0047 cB | 0.052 ± 0.0069 cB | |
| Baking | 0.25 ± 0.0074 aB | 0.089 ± 0.0078 bB | |
| Steaming | 0.081 ± 0.0066 bB | 0.12 ± 0.0056 aB | |
| Spices | 0.076 | <0.001 | |
| Stages | <0.001 | <0.001 | |
| Spices * Stages | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, H.; Zhu, Y.; Feng, C.; He, Z.; Chen, J.; Zeng, M. Processing Stage-Induced Formation of Advanced Glycation End Products in Cooked Sausages with the Addition of Spices. Foods 2023, 12, 3788. https://doi.org/10.3390/foods12203788
Li Y, Li H, Zhu Y, Feng C, He Z, Chen J, Zeng M. Processing Stage-Induced Formation of Advanced Glycation End Products in Cooked Sausages with the Addition of Spices. Foods. 2023; 12(20):3788. https://doi.org/10.3390/foods12203788
Chicago/Turabian StyleLi, Yong, Hua Li, Yinchun Zhu, Cuiping Feng, Zhiyong He, Jie Chen, and Maomao Zeng. 2023. "Processing Stage-Induced Formation of Advanced Glycation End Products in Cooked Sausages with the Addition of Spices" Foods 12, no. 20: 3788. https://doi.org/10.3390/foods12203788