Ultrasound-Assisted Extraction of Bioactive Compounds from Cocoa Shell and Their Encapsulation in Gum Arabic and Maltodextrin: A Technology to Produce Functional Food Ingredients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. RSM Analysis and Optimizing UAE Extraction
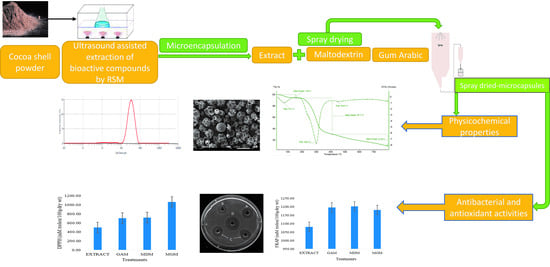
2.3. Microencapsulation Experiment
2.4. Determination of Bioactive Compounds (TPC and TFC) and Antioxidant Activity (DPPH and FRAP) in CSP Microcapsules
2.5. Physicochemical Properties of Microcapsules
2.6. Antimicrobial Activity Experiment
2.6.1. Growth Condition
2.6.2. Antimicrobial Screening by Disc Diffusion Method
2.6.3. Minimum Inhibitory Concentration (MIC)
2.7. Thermogravimetric Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Ultrasound-Assisted Extraction (UAE) Using RSM
3.2. Antimicrobial Activity by Disk Diffusion Assay and Minimum Inhibitory Concentration (MIC)
3.3. Effects of Microencapsulation on Functional Properties of CSP
3.4. Quality Characteristics of Microcapsules
3.5. Microcapsule’s Surface Structure Characterization and Particle Size Distribution
3.6. Analyses of Thermogravimetry
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Segovia, Á.; Bartolomé, B.; Aguilera, Y. Extraction of phenolic compound from cocoa shell: Modeling using response surface methodology and artificial neural networks. Sep. Purif. Technol. 2021, 270, 118779. [Google Scholar] [CrossRef]
- Md Yusof, A.H.; Abd Gani, S.S.; Zaidan, U.H.; Halmi, M.I.E.; Zainudin, B.H. Optimization of an ultrasound-assisted extraction condition for flavonoid compounds from cocoa shells (Theobroma cacao) using response surface methodology. Molecules 2019, 24, 711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, I.D.; Okiyama, D.C.G.; da Costa Rodrigues, C.E. Simultaneous green extraction of fat and bio-active compounds of cocoa shell and protein fraction functionalities evaluation. Food Res. Intl. 2020, 137, 109622. [Google Scholar] [CrossRef] [PubMed]
- Cisowska, J.K.; Szczepaniak, O.; Powałowska, D.S.; Piechocka, J.; Szulc, P.; Dziedziński, M. Antioxidant potential of various solvent extract from Morus alba fruits and its major polyphenols composition. Ciencia Rural. 2020, 50, 17. [Google Scholar]
- Yekdane, N.; Goli, S.A.H. Effect of pomegranate juice on characteristics and oxidative stability of microencapsulated pomegranate seed oil using spray drying. Food Bioprocess Technol. 2019, 12, 1614–1625. [Google Scholar] [CrossRef]
- Insang, S.; Kijpatanasilp, I.; Jafari, S.; Assatarakul, K. Ultrasound-assisted extraction of functional compound from mulberry (Morus alba L.) leaf using response surface methodology and effect of microencapsulation by spray drying on quality of optimized extract. Ultrason. Sonochem. 2021, 82, 105806. [Google Scholar] [CrossRef] [PubMed]
- Speranza, B.; Petruzzi, L.; Bevilacqua, A.; Gallo, M.; Campaniello, D.; Corbo, M.R. Encapsulation of active compounds in fruit and vegetable juice processing: Current state and perspectives. J. Food Sci. 2017, 82, 1291–1301. [Google Scholar] [CrossRef] [Green Version]
- Jafari, S.; Jafari, S.M.; Ebrahimi, M.; Kijpatanasilp, I.; Assatarakul, K. A decade overview and prospect of spray drying encapsulation of bioactives from fruit products: Characterization, food application and in vitro gastrointestinal digestion. Food Hydrocoll. 2022, 134, 108068. [Google Scholar] [CrossRef]
- Furuta, T.; Neoh, T.L. Spray Drying Encapsulation of Bioactive Ingredients: An Overview of Various Aspects. Spray Drying Encapsulation of Bioactive. Materials. 2021. [Google Scholar] [CrossRef]
- Sarabandi, K.; Jafari, S.M.; Mahoonak, A.S.; Mohammadi, A. Application of gum Arabic and maltodextrin for encapsulation of eggplant peel extract as a natural antioxidant and color source. Int. J. Biol. Macromol. 2019, 140, 59–68. [Google Scholar] [CrossRef]
- Rajabi, H.; Ghorbani, M.; Jafari, S.M.; Mahoonak, A.S.; Rajabzadeh, G. Retention of saffron bioac-tive components by spray drying encapsulation using maltodextrin, gum Arabic and gelatin as wall materials. Food Hydrocoll. 2015, 51, 327–337. [Google Scholar] [CrossRef]
- Ramakrishnan, Y.; Adzahan, N.M.; Yusof, Y.A.; Muhammad, K. Effect of wall materials on the spray drying efficiency, powder properties and stability of bioactive compounds in tamarillo juice microencapsulation. Powder Technol. 2018, 328, 406–414. [Google Scholar] [CrossRef]
- Saénz, C.; Tapia, S.; Chávez, J.; Robert, P. Microencapsulation by spray drying of bioactive com-pounds from cactus pear (Opuntia ficus-indica). Food Chem. 2009, 114, 616–622. [Google Scholar] [CrossRef]
- Sirichan, T.; Kijpatanasilp, I.; Asadatorn, N.; Assatarakul, K. Optimization of ultrasound extrac-tion of functional compound from makiang seed by response surface methodology and antimicrobial activity of optimized extract with its application in orange juice. Ultrason. Sonochem. 2022, 83, 105916. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Pongsarn, K.; Srestasupana, C.; Wetchasart, N.; Assatarakul, K. Kinetic study of microbial inhibition by dimethyl dicarbonate and quality attributes of pomegranate juice during cold storage. Ultrason. Sonochem. 2021, 152, 112309. [Google Scholar] [CrossRef]
- Jafari, S.; Rungroj, N.; Worobo, R.W.; Assatarakul, K. Kinetic study of selected microorganisms and quality attributes during cold storage of mango and passion fruit smoothie subjected to dimethyl dicarbonate. Intl. J. Food Microbiol. 2021, 358, 109404. [Google Scholar] [CrossRef]
- Jafari, S.; Thongmat, K.; Kijpatanasilp, I.; Kerdsup, P.; Naknaen, P.; Taweechotipatr, M.; Assatarakul, K. Phenolic compound profile of probiotic (Lacticaseibacillusrhamnosus LR5) fortified vegetable tablet and probiotic survival in the simulated gastrointestinal tract. Sci. Rep. 2022, 12, 1014. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, M.; Frassinetti, S.; Caltavuturo, L.; Montero, L.; Dinelli, G.; Longo, V.; Pucci, L. Citrus bergamia powder: Antioxidant, antimicrobial and anti-inflammatory properties. J. Funct. Foods. 2017, 31, 255–265. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Palma, M.; Barroso, C. Pressurized liquid extraction of isoflavones from soybeans. Anal. Chim. Acta 2004, 522, 169–177. [Google Scholar] [CrossRef]
- Chen, X.; Ding, J.; Ji, D.; He, S.; Ma, H. Optimization of ultrasonic-assisted extraction conditions for bioactive components from coffee leaves using the Taguchi design and response surface methodology. J. Food Sci. 2020, 85, 1742–1751. [Google Scholar] [CrossRef]
- Sungthong, B.; Phadungkit, M. Anti-tyrosinase and DPPH radical scavenging activities of selected Thai herbal extracts traditionally used as skin toner. Pharmacogn. J. 2015, 7, 97–101. [Google Scholar] [CrossRef]
- Akowuah, G.A.; Mariam, A.; Chin, J.H. The effect of extraction temperature on total phenols and anti-oxidant activity of Gynura procumbens leaf. Pharmacogn Mag. 2009, 5, 81. [Google Scholar]
- Burgut, A. Growth inhibition of foodborne pathogens by co-microencapsulation of lactobacilli cell free and propolis extracts. J. Food Saf. 2019, 41, e12863. [Google Scholar] [CrossRef]
- Başyiğit, B.; Sağlam, H.; Kandemir, Ş.; Karaaslan, A.; Karaaslan, M. Microencapsulation of sour cherry oil by spray drying: Evaluation of physical morphology, thermal properties, storage stability, and atimicrobial activity. Powder Technol. 2020, 364, 654–663. [Google Scholar] [CrossRef]
- Karaaslan, M.; Şengün, F.; Cansu, Ü.; Başyiğit, B.; Sağlam, H.; Karaaslan, A. Gum arabic/maltodextrin microencapsulation confers peroxidation stability and antimicrobial ability to pepper seed oil. Food Chem. 2021, 337, 127748. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Paim, D.R.; Costa, S.D.; Walter, E.H.; Tonon, R.V. Microencapsulation of probiotic jussara (Euterpe edulis M.) juice by spray drying. LWT 2016, 74, 21–25. [Google Scholar] [CrossRef]
- Tomsone, L.; Galoburda, R.; Kruma, Z.; Durrieu, V.; Cinkmanis, I. Microencapsulation of horseradish (Armoracia rusticana L.) juice using spray-drying. Foods 2020, 9, 1332. [Google Scholar] [CrossRef]
- Nambiar, R.B.; Sellamuthu, P.S.; Perumal, A.B. Microencapsulation of tender coconut water by spray drying: Effect of Moringa oleifera gum, maltodextrin concentrations, and inlet temperature on powder qualities. Food Bioprocess Technol. 2017, 10, 1668–1684. [Google Scholar] [CrossRef]
- Janiszewska, E.; Witrowa-Rajchert, D. The influence of powder morphology on the effect of rosemary aroma microencapsulation during spray drying. Intl. J. Food Sci. Technol. 2009, 44, 2438–2444. [Google Scholar] [CrossRef]
- Colín-Cruz, M.A.; Pimentel-González, D.J.; Carrillo-Navas, H.; Alvarez-Ramírez, J.; Guadarrama-Lezama, A.Y. Co-encapsulation of bioactive compounds from blackberry juice and probiotic bacteria in biopolymeric matrices. LWT 2019, 110, 94–101. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Marconi Germer, S.P.; Alvim, I.D.; de Aguirre, J.M. Storage stability of spray-dried blackberry powder produced with maltodextrin or gum Arabic. Drying Technol. 2013, 31, 470–478. [Google Scholar] [CrossRef]








| Independent Variables | Code Symbols | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Concentration (%) | X1 | 60 | 80 | 100 |
| Temperature (°C) | X2 | 45 | 55 | 65 |
| Time (min) | X3 | 30 | 45 | 60 |
| Independent Variables | Responses | ||||||
|---|---|---|---|---|---|---|---|
| Run | Concentration (%) | Temperature (°C) | Time (min) | TPC (mg GAE/100g dry wt) | TFC (mg QE/100g dry wt) | DPPH (mM Trolox/100g dry wt) | FRAP (mM Trolox/100g dry wt) |
| 1 | 60 | 55 | 45 | 140.50 | 84.37 | 522.38 | 1134.53 |
| 2 | 60 | 65 | 45 | 160.12 | 74.95 | 450.00 | 1197.68 |
| 3 | 60 | 55 | 30 | 97.22 | 67.93 | 424.25 | 980.21 |
| 4 | 60 | 55 | 45 | 100.25 | 99.26 | 480.00 | 1098.20 |
| 5 | 60 | 55 | 45 | 150.70 | 88.58 | 530.00 | 1000.58 |
| 6 | 80 | 45 | 60 | 105.98 | 76.85 | 438.00 | 926.11 |
| 7 | 100 | 45 | 30 | 45.69 | 41.07 | 246.13 | 479.78 |
| 8 | 80 | 65 | 30 | 102.26 | 65.48 | 353.63 | 978.74 |
| 9 | 60 | 55 | 60 | 150.38 | 86.20 | 448.00 | 1197.68 |
| 10 | 80 | 55 | 45 | 119.92 | 78.64 | 502.00 | 951.89 |
| 11 | 80 | 45 | 30 | 87.30 | 52.93 | 319.25 | 885.00 |
| 12 | 60 | 55 | 45 | 135.90 | 84.23 | 393.63 | 951.37 |
| 13 | 100 | 65 | 30 | 63.71 | 53.74 | 398.63 | 679.00 |
| 14 | 100 | 45 | 60 | 104.40 | 69.64 | 495.50 | 785.00 |
| 15 | 80 | 65 | 60 | 132.56 | 57.43 | 320.50 | 959.79 |
| 16 | 60 | 45 | 45 | 110.22 | 77.00 | 512.00 | 1024.00 |
| 17 | 100 | 55 | 45 | 53.92 | 52.34 | 509.34 | 820.00 |
| TPC (mg GAE/100g dry wt) | TFC (mg QE/100g dry wt) | DPPH (mM Trolox/100 g dry wt) | FRAP (mM Trolox/100g dry wt) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | Sum of Squares | df | Mean Squares | p-Value | Sum of Squares | df | Mean Squares | p-Value | Sum of Squares | df | Mean Squares | p-Value | Sum of Squares | df | Mean Squares | p-Value |
| Model | 3538.27 | 9 | 1770.20 | 0.0246 | 93,693.10 | 9 | 393.14 | 0.0055 | 15,931.78 | 9 | 10,410.34 | 0.0330 | 4.625 | 9 | 51,392.22 | 0.0202 |
| X1 | 725.39 | 1 | 5580.32 | 0.0058 | 996.45 | 1 | 725.39 | 0.0059 | 5580.32 | 1 | 996.45 | 0.5398 | 1.965 | 1 | 1.965 | 0.0029 |
| X2 | 39.98 | 1 | 1224.16 | 0.1096 | 960.29 | 1 | 39.98 | 0.3909 | 1224.16 | 1 | 960.29 | 0.5471 | 34,191.45 | 1 | 34,191.45 | 0.1047 |
| X3 | 261.12 | 1 | 1089.46 | 0.1276 | 25,346.69 | 1 | 261.12 | 0.0521 | 1089.46 | 1 | 25,346.69 | 0.0140 | 16,889.82 | 1 | 16,889.82 | 0.2316 |
| X1X2 | 22.90 | 1 | 663.85 | 0.2193 | 2977.05 | 1 | 22.90 | 0.5112 | 663.85 | 1 | 2977.05 | 0.3021 | 697.09 | 1 | 697.09 | 0.7978 |
| X1X3 | 35.87 | 1 | 26.87 | 0.7939 | 3707.12 | 1 | 35.87 | 0.4151 | 26.87 | 1 | 3707.12 | 0.2539 | 449.72 | 1 | 449.72 | 0.8369 |
| X2X3 | 509.21 | 1 | 85.77 | 0.6425 | 11,210.95 | 1 | 509.21 | 0.0138 | 85.77 | 1 | 11,210.95 | 0.0674 | 5530.83 | 1 | 5530.83 | 0.4780 |
| X12 | 27.07 | 1 | 225.01 | 0.4580 | 1690.84 | 1 | 27.07 | 0.4763 | 225.01 | 1 | 1690.84 | 0.4289 | 8732.85 | 1 | 8732.85 | 0.3777 |
| X22 | 139.45 | 1 | 115.05 | 0.5919 | 2849.84 | 1 | 139.45 | 0.1314 | 115.05 | 1 | 2849.84 | 0.3118 | 76.68 | 1 | 76.68 | 0.9322 |
| X32 | 100.77 | 1 | 59.94 | 0.6973 | 15,945.32 | 1 | 100.77 | 0.1899 | 59.94 | 1 | 15,945.32 | 0.0366 | 1964.34 | 1 | 1964.34 | 0.6686 |
| Residual | 334.69 | 7 | 364.79 | 16,793.65 | 7 | 47.81 | 2553.52 | 7 | 2399.09 | 68,924.78 | 7 | 9846.40 | ||||
| Lack of Fit | 185.11 | 4 | 277.10 | 0.7029 | 5046.83 | 4 | 46.28 | 0.5467 | 1108.41 | 4 | 1261.71 | 0.8487 | 47,344.69 | 4 | 11,836.17 | 0.3557 |
| Pure Error | 149.59 | 3 | 481.70 | 11,746.81 | 3 | 49.86 | 1445.11 | 3 | 3915.60 | 21,580.10 | 3 | 7193.37 | ||||
| Cor Total | 3872.96 | 16 | 1.105 | 16 | 18,485.29 | 16 | 5.315 | 16 | ||||||||
| C.V.% | 17.46 | 9.70 | 11.34 | 10.51 | ||||||||||||
| R2 | 0.86 | 0.91 | 0.84 | 0.87 | ||||||||||||
| Adj-R2 | 0.68 | 0.80 | 0.65 | 0.70 | ||||||||||||
| Bioactive Properties | ||||
|---|---|---|---|---|
| Factor | TPC | TFC | DPPH | FRAP |
| Intercept | 97.91 * | 72.98 * | 439.52 * | 928.31 * |
| Linear | ||||
| X1 | −30.06 * | −10.84 * | −12.70 | −178.40 * |
| X2 | 13.03 | 2.36 | 11.54 | 68.88 |
| X3 | 34.25 | 16.77 | 165.23 * | 134.87 |
| Cross product | ||||
| X1X2 | −13.35 | −2.48 | 28.26 | −13.68 |
| X1X3 | −4.03 | −4.65 | 47.31 | 16.48 |
| X2X3 | −6.11 | −14.90 * | −69.90 | −49.10 |
| Quadratic | ||||
| X12 | −9.34 | −3.24 | 25.59 | −58.16 |
| X22 | 6.60 | −7.27 | −32.87 | −5.39 |
| X32 | −10.72 | −13.91 | −174.92 * | −61.39 |
| Treatments | |||||
|---|---|---|---|---|---|
| Parameters | GAM | MDM | GMM | SEM | p-Value |
| Encapsulation efficiency (%) ns | 67.05 ± 0.37 | 62.95 ± 1.98 | 59.92 ± 0.44 | 0.973 | 1.020 |
| Moisture content (%) ns | 2.72 ± 0.01 | 2.56 ± 0.07 | 2.69 ± 0.07 | 0.012 | 3.090 |
| Water activity ns | 0.13 ± 0.00 | 0.15 ± 0.00 | 0.10 ± 0.00 | 0.003 | 3.900 |
| Solubility (%) ns | 75.99 ± 0.14 | 75.94 ± 0.06 | 75.50 ± 0.55 | 0.073 | 0.359 |
| Color values ns | |||||
| L* | 66.46 ± 0.00 | 60.39 ± 0.00 | 63.79 ± 0.07 | 0.827 | 1.330 |
| a* | 1.00 ± 0.00 | 1.14 ± 0.00 | 1.09 ± 0.00 | 0.018 | 1.930 |
| b* | 14.11 ± 0.00 | 9.91 ± 0.00 | 11.92 ± 0.00 | 0.573 | 3.960 |
| Particle size (μm) ns | |||||
| D (4,3) | 31.00 ± 0.06 | 31.90 ± 0.01 | 34.30 ± 0.03 | 0.464 | 1.180 |
| D (3,2) | 15.50 ± 0.02 | 14.60 ± 0.01 | 18.50 ± 0.04 | 0.555 | 1.730 |
| Microorganism | ||||
|---|---|---|---|---|
| Sample/Parameter | Bacillus subtilis | Escherichia coli | Salmonella Typhimurium | Staphylococcus aureus |
| Inhibition zone (mm) | ||||
| CSP extract | 9.4 ± 0.20 a | 11.3 ± 0.50 a | 8.9 ± 0.75 b | 10.9 ± 0.20 a |
| GAM (5 mg/mL) | 7.1 ± 0.35 c | 10.05 ± 0.10 b | 7.2 ± 0.20 c | 9.8 ± 0.20 b |
| MDM (5 mg/mL) | 8.4 ± 0.15 b | 8.9 ± 1.20 c | 8.1 ± 0.34 c | 8.5 ± 0.25 c |
| GMM (5 mg/mL) | 9.6 ± 0.15 a | 11.5 ± 0.34 a | 9.0 ± 0.50 b | 11.1 ± 0.45 a |
| Chloramphenicol (30 µg/mL) | 9.5 ± 0.20 a | 11.2 ± 0.30 a | 11.0 ± 0.50 a | 9.1 ± 0.16 b |
| MIC (mg/mL) | ||||
| CSP extract | 1.80 b | 1.65 b | 1.90 b | 1.50 b |
| GAM | 2.85 a | 2.00 a | 2.85 a | 2.00 a |
| MDM | 2.25 a | 2.50 a | 2.25 a | 2.50 a |
| GMM | 1.85 b | 1.50 b | 1.85 b | 1.50 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafari, S.; Karami, Z.; Shiekh, K.A.; Kijpatanasilp, I.; Worobo, R.W.; Assatarakul, K. Ultrasound-Assisted Extraction of Bioactive Compounds from Cocoa Shell and Their Encapsulation in Gum Arabic and Maltodextrin: A Technology to Produce Functional Food Ingredients. Foods 2023, 12, 412. https://doi.org/10.3390/foods12020412
Jafari S, Karami Z, Shiekh KA, Kijpatanasilp I, Worobo RW, Assatarakul K. Ultrasound-Assisted Extraction of Bioactive Compounds from Cocoa Shell and Their Encapsulation in Gum Arabic and Maltodextrin: A Technology to Produce Functional Food Ingredients. Foods. 2023; 12(2):412. https://doi.org/10.3390/foods12020412
Chicago/Turabian StyleJafari, Saeid, Zohreh Karami, Khursheed Ahmad Shiekh, Isaya Kijpatanasilp, Randy W. Worobo, and Kitipong Assatarakul. 2023. "Ultrasound-Assisted Extraction of Bioactive Compounds from Cocoa Shell and Their Encapsulation in Gum Arabic and Maltodextrin: A Technology to Produce Functional Food Ingredients" Foods 12, no. 2: 412. https://doi.org/10.3390/foods12020412