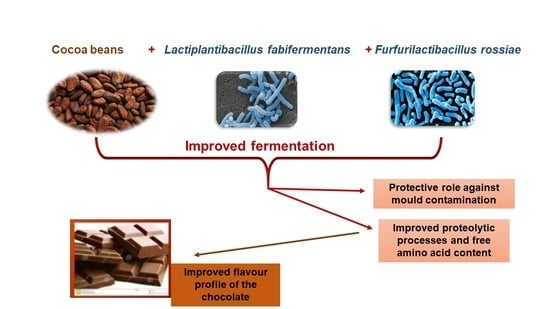
Fine Cocoa Fermentation with Selected Lactic Acid Bacteria: Fermentation Performance and Impact on Chocolate Composition and Sensory Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain Growth and Maintenance
2.2. Fermentation Setup and Control
2.3. Mould Growth Inhibition
2.4. Protein Extraction and Electrophoretic Characterisation
2.5. Chromatographic Characterisations
2.6. Free Amino Acids Profile
2.7. Cocoa Liquor and Chocolate Preparation
2.8. Volatile Molecules Assay
2.9. Sensory Analysis of Chocolate
2.10. Statistical Analysis
3. Results
3.1. Fermentation Parameters
3.2. Mould Growth Inhibition
3.3. Protein and Peptide Characterisation
3.4. Free Amino Acid Profile
3.5. Volatile Molecules of Cocoa Bulk
3.6. Sensory Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Vuyst, L.; Weckx, S. The cocoa bean fermentation process: From ecosystem analysis to starter culture development. J. Appl. Microbiol. 2016, 121, 5–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vuyst, L.; Leroy, F. Functional role of yeasts, lactic acid bacteria and acetic acid bacteria in cocoa fermentation processes. FEMS Microbiol. Rev. 2020, 44, 432–453. [Google Scholar] [CrossRef] [PubMed]
- United Nations. International Cocoa Agreement. Treaty Series, 2871, 3. TD/COCOA.10/3. 2010. Available online: https://treaties.un.org/ (accessed on 10 February 2022).
- ICCO. Fine or Flavor Cocoa. Available online: https://www.icco.org/fine-or-flavor-cocoa/ (accessed on 9 November 2021).
- Kadow, D.; Bohlmann, J.; Phillips, W.; Lieberei, R. Identification of main fine flavour components in two genotypes of the cocoa tree (Theobroma cacao L.). J. Appl. Bot. Food Qual. 2013, 86, 90–98. [Google Scholar] [CrossRef]
- Crafack, M.; Mikkelsen, M.B.; Saerens, S.; Knudsen, M.; Blennow, A.; Lowor, S.; Takrama, J.; Swiegers, J.H.; Petersen, G.B.; Heimdal, H.; et al. Influencing cocoa flavour using Pichia kluyveri and Kluyveromyces marxianus in a defined mixed starter culture for cocoa fermentation. Int. J. Food Microbiol. 2013, 167, 103–116. [Google Scholar] [CrossRef] [PubMed]
- de C. Lima, C.O.; Vaz, A.B.M.; De Castro, G.M.; Lobo, F.; Solar, R.; Rodrigues, C.; Martins Pinto, L.R.; Vandenberghe, L.; Pereira, G.; Miúra da Costa, A.; et al. Integrating microbial metagenomics and physicochemical parameters and a new perspective on starter culture for fine cocoa fermentation. Food Microbiol. 2021, 93, 103608. [Google Scholar] [CrossRef]
- Lefeber, T.; Papalexandratou, Z.; Gobert, W.; Camu, N.; De Vuyst, L. On-farm implementation of a starter culture for improved cocoa bean fermentation and its influence on the flavour of chocolates produced thereof. Food Microbiol. 2012, 30, 379–392. [Google Scholar] [CrossRef]
- Magalhães da Veiga Moreira, I.; de Figueiredo Vilela, L.; da Cruz Pedroso Miguel, M.; Santos, C.; Lima, N.; Freitas Schwan, R. Impact of a microbial cocktail used as a starter culture on cocoa fermentation and chocolate flavor. Molecules 2017, 22, 766. [Google Scholar] [CrossRef] [Green Version]
- Pereira, G.V.; Miguel, M.G.; Ramos, C.L.; Schwan, R.F. Microbiological and physicochemical characterization of small-scale cocoa fermentations and screening of yeast and bacterial strains to develop a defined starter culture. Appl. Environ. Microbiol. 2012, 78, 5395–5405. [Google Scholar] [CrossRef] [Green Version]
- Korcari, D.; Ricci, G.; Fanton, A.; Emide, D.; Barbiroli, A.; Fortina, M.G. Exploration of Lactiplantibacillus fabifermentans and Furfurilactobacillus rossiae as potential cocoa fermentation starters. J. Appl. Microbiol. 2022, 133, 1769–1780. [Google Scholar] [CrossRef]
- Kumari, N.; Kofi, K.J.; Grimbs, S.; D’Souza, R.N.; Kuhnert, N.; Vrancken, G.; Ullrich, M.S. Biochemical fate of vicilin storage protein during fermentation and drying of cocoa beans. Food Res. Int. 2016, 90, 53–65. [Google Scholar] [CrossRef]
- Hogenboom, J.A.; D’Incecco, P.; Fuselli, F.; Pellegrino, L. Ion-exchange chromatographic method for the determination of the free amino acid composition of cheese and other dairy products: An inter-laboratory validation study. Food Anal. Met. 2017, 10, 3137–3148. [Google Scholar] [CrossRef] [Green Version]
- ISO 13299; Sensory Analysis—Methodology—General Guidance to Establish a Sensory Profile. International Organization for Standardization: Geneva, Switzerland, 2010.
- ISO 8589; Sensory Analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization: Geneva, Switzerland, 2007.
- Macfie, H.J.H.; Bratchell, N.; Greenhoff, K.; Vallis, L.V. Designs to balance the effect of order of presentation and first-order carry-over effects in hall tests. J. Sens. Stud. 1989, 4, 129–148. [Google Scholar] [CrossRef]
- Ouattara, H.D.; Ouattara, H.G.; Droux, M.; Reverchon, S.; Nasser, W.; Niamke, S.L. Lactic acid bacteria involved in cocoa beans fermentation from Ivory Coast: Species diversity and citrate lyase production. Int. J. Food Microbiol. 2017, 256, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Lerceteau, E.; Rogers, J.; Pétiard, V.; Crouzillat, D. Evolution of cacao bean proteins during fermentation: A study by two-dimensional electrophoresis. J. Sci. Food Agricul. 1999, 79, 619–625. [Google Scholar] [CrossRef]
- De Vuyst, L.; Lefeber, T.; Papalexandratou, Z.; Camu, N. The functional role of lactic acid bacteria in cocoa bean fermentation. In Biotechnology of Lactic Acid Bacteria: Novel Applications; Mozzi, F., Raya, R.R., Vignolo, G.M., Eds.; Wiley-Blackwell: Ames, IA, USA, 2010; pp. 301–326. [Google Scholar] [CrossRef]
- Hinneh, M.; Semanhyia, E.; Van de Walle, D.; De Winne, A.; Tzompa-Sosa, D.A.; Scalone, G.L.; De Meulenaer, B.; Messens, K.; Van Durme, J.; Afoakwa, E.O.; et al. Assessing the influence of pod storage on sugar and free amino acid profiles and the implications on some Maillard reaction related flavor volatiles in Forastero cocoa beans. Food Res. Int. 2018, 111, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.; Biehl, B.; Wazir, S.K.S. The major seed proteins of Theobroma cacao L. Food Chem. 1993, 47, 145–151. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavor formation and character in cocoa and chocolate: A critical review. Crit. Rev. Food Sci. Nutr. 2008, 48, 840–857. [Google Scholar] [CrossRef]
- Rawel, H.; Huschek, G.; Sagu, S.; Homann, T. Cocoa bean proteins: Characterization, changes and modifications due to ripening and post-harvest processing. Nutrients 2019, 11, 428. [Google Scholar] [CrossRef] [Green Version]
- Kirchhoff, P.-M.; Biehl, B.; Ziegeler-Berghausen, H.; Hammoor, M.; Lieberei, R. Kinetics of the formation of free amino acids in cocoa seeds during fermentation. Food Chem. 1989, 34, 161–179. [Google Scholar] [CrossRef]
- Kirchhoff, P.-M.; Biehl, B.; Crone, G. Peculiarity of the accumulation of free amino acids during cocoa fermentation. Food Chem. 1989, 31, 295–311. [Google Scholar] [CrossRef]
- Deus, V.L.; Bispo, E.S.; Franca, A.S.; Gloria, M.B.A. Understanding amino acids and bioactive amines changes during on-farm cocoa fermentation. J. Food Comp. Anal. 2021, 97, 103776. [Google Scholar] [CrossRef]
- Romanens, E.; Freimüller Leischtfeld, S.; Volland, A.; Stevens, M.J.A.; Krähenmann, U.; Isele, D.; Fischer, B.; Meile, L.; Miescher Schwenninger, S. Screening of lactic acid bacteria and yeast strains to select adapted anti-fungal co-cultures for cocoa bean fermentation. Int. J. Food Microbiol. 2019, 290, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Essia Ngang, J.-J.; Yadang, G.; Sado Kamdem, S.L.; Kouebou, C.P.; Youte Fanche, S.A.; Tsochi Kougan, D.L.; Tsoungui, A.; Etoa, F.-X. Antifungal properties of selected lactic acid bacteria and application in the biological control of ochratoxin A producing fungi during cocoa fermentation. Biocontrol. Sci. Technol. 2014, 25, 245–259. [Google Scholar] [CrossRef]
- Ruggirello, M.; Nucera, D.; Cannoni, M.; Peraino, A.; Rosso, F.; Fontana, M.; Cocolin, L.; Dolci, P. Antifungal activity of yeasts and lactic acid bacteria isolated from cocoa bean fermentations. Food Res. Int. 2019, 115, 519–525. [Google Scholar] [CrossRef]






| Sample | Fermentation Protocol | Inoculum (CFU/g of Cocoa) |
|---|---|---|
| Control | Spontaneous fermentation | - |
| A | Inoculum with strain L. fabifermentans SAF13 at t0 | 6.25 × 106 |
| B | Inoculum with strain L. fabifermentans SAF13 at t0 and t48 | 6.25 × 106 at t0 1.6 × 107 at t48 |
| C | Inoculum with strain F. rossiae SAF51 at t0 | 1.4 × 107 |
| D | Inoculum with strain F. rossiae SAF51 at t0 and t48 | 1.4 × 107 at t0 1.9 × 107 at t48 |
| E | Inoculum with strains L. fabifermentans SAF13 and F. rossiae SAF51 at t0 and t48 | SAF13: 6.25 × 106 at t0 +1.6 × 107 at t48 SAF51: 1.4 × 107 at t0 + 1.9 × 107 at t48 |
| Sample | Unfermented | Control | A | B | C | D | E |
|---|---|---|---|---|---|---|---|
| Asp | 2.21 ± 0.02 a | 3.13 ± 0.03 b | 3.20 ± 0.03 b | 3.30 ± 0.03 bc | 3.70 ± 0.04 cd | 3.54 ± 0.04 bcd | 3.94 ± 0.04 d |
| Thr | 0.37 ± 0.01 a | 2.29 ± 0.03 b | 2.21 ± 0.02 b | 2.12 ± 0.02 b | 2.51 ± 0.03 bc | 2.41 ± 0.03 bc | 2.74 ± 0.03 c |
| Ser | 0.78 ± 0.01 a | 2.49 ± 0.03 bc | 2.35 ± 0.03 b | 2.27 ± 0.03 b | 2.82 ± 0.04 cd | 2.50 ± 0.03 bc | 3.02 ± 0.04 d |
| Asn | 4.14 ± 0.06 abc | 3.74 ± 0.05 a | 4.49 ± 0.06 c | 4.32 ± 0.06 bc | 3.72 ± 0.05 a | 3.96 ± 0.06 ab | 3.92 ± 0.05 ab |
| Glu | 4.68 ± 0.05 a | 4.91 ± 0.06 ab | 5.40 ± 0.06 c | 5.52 ± 0.06 cd | 5.24 ± 0.06 bc | 5.52 ± 0.06 cd | 5.85 ± 0.07 d |
| Gln | 0.98 ± 0.07 b | 0.58 ± 0.04 ab | 0.51 ± 0.04 a | 0.38 ± 0.03 a | 0.48 ± 0.04 a | 0.41 ± 0.03 a | 0.43 ± 0.03 a |
| Gly | 0.32 ± 0.01 a | 1.07 ± 0.01 bc | 1.06 ± 0.01 bc | 0.92 ± 0.01 b | 1.41 ± 0.02 c | 1.20 ± 0.02 bc | 1.33 ± 0.02 bc |
| Ala | 1.46 ± 0.02 a | 4.43 ± 0.07 b | 4.62 ± 0.07 bc | 4.80 ± 0.07 b | 4.95 ± 0.07 c | 4.89 ± 0.07 c | 4.81 ± 0.07 b |
| Val | 1.24 ± 0.01 a | 4.05 ± 0.04 cd | 3.74 ± 0.04 bc | 3.53 ± 0.04 b | 4.68 ± 0.05 ef | 4.31 ± 0.05 de | 5.03 ± 0.06 f |
| Met | 0.06 ± 0.01 ab | 0.79 ± 0.02 de | 0.49 ± 0.01 bcd | 0.28 ± 0.01 abc | 1.22 ± 0.03 e | 0.7 ± 0.02 cd | 1.07 ± 0.03 de |
| Ile | 0.97 ± 0.02 a | 2.42 ± 0.05 bc | 2.17 ± 0.05 b | 2.07 ± 0.04 b | 2.92 ± 0.06 de | 2.65 ± 0.06 cd | 3.24 ± 0.07 e |
| Leu | 1.30 ± 0.03 a | 7.91 ± 0.17 bc | 7.49 ± 0.16 b | 7.80 ± 0,17 bc | 8.52 ± 0.19 de | 8.17 ± 0.18 cd | 8.74 ± 0.19 e |
| Tyr | 1.77 ± 0.11 a | 4.35 ± 0.26 bc | 3.97 ± 0.24 b | 4.06 ± 0.24 b | 5.30 ± 0.32 d | 4.63 ± 0.28 c | 5.66 ± 0.34 d |
| Phe | 1.28 ± 0.02 a | 6.78 ± 0.09 c | 6.29 ± 0.08 b | 6.21 ± 0.08 b | 7.93 ± 0.11 d | 6.77 ± 0.09 c | 8.12 ± 0.11 d |
| Gaba | 4.77 ± 0.33 d | 4.45 ± 0.31 cd | 3.74 ± 0.26 a | 3.95 ± 0.27 ab | 4.63 ± 0.32 cd | 4.27 ± 0.30 bc | 4.74 ± 0.33 d |
| Orn | 0.21 ± 0.01 a | 0.12 ± 0.01 a | 0.17 ± 0.01 a | 0.12 ± 0.01 a | 0.27 ± 0.01 a | 0.30 ± 0.01 a | 0.27 ± 0.01 a |
| Lys | 0.76 ± 0.01 a | 4.40 ± 0.04 c | 3.93 ± 0.04 b | 3.57 ± 0.03 b | 5.32 ± 0.05 e | 4.74 ± 0.05 cd | 5.16 ± 0.05 de |
| His | 2.57 ± 0.04 c | 2.23 ± 0.04 abc | 1.82 ± 0.03 a | 1.82 ± 0.03 a | 2.32 ± 0.04 bc | 1.99 ± 0.03 ab | 2.41 ± 0.04 bc |
| Arg | 0.29 ± 0.01 a | 0.26 ± 0.01 a | 0.20 ± 0.01 a | 0.14 ± 0.01 a | 0.13 ± 0.01 a | 0.15 ± 0.01 a | 0.19 ± 0.01 a |
| Pro | 1.34 ± 0.03 a | 2.19 ± 0.04 bc | 2.15 ± 0.04 bc | 1.80 ± 0.04 b | 2.40 ± 0.05 cd | 2.04 ± 0.04 bc | 2.82 ± 0.06 d |
| Total | 31.50 ± 0.27 a | 62.59 ± 0.53 d | 60.00 ± 0.51 c | 58.98 ± 0.50 b | 70.47 ± 0.60 f | 65.15 ± 0.55 e | 73.49 ± 0.62 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korcari, D.; Fanton, A.; Ricci, G.; Rabitti, N.S.; Laureati, M.; Hogenboom, J.; Pellegrino, L.; Emide, D.; Barbiroli, A.; Fortina, M.G. Fine Cocoa Fermentation with Selected Lactic Acid Bacteria: Fermentation Performance and Impact on Chocolate Composition and Sensory Properties. Foods 2023, 12, 340. https://doi.org/10.3390/foods12020340
Korcari D, Fanton A, Ricci G, Rabitti NS, Laureati M, Hogenboom J, Pellegrino L, Emide D, Barbiroli A, Fortina MG. Fine Cocoa Fermentation with Selected Lactic Acid Bacteria: Fermentation Performance and Impact on Chocolate Composition and Sensory Properties. Foods. 2023; 12(2):340. https://doi.org/10.3390/foods12020340
Chicago/Turabian StyleKorcari, Dea, Alberto Fanton, Giovanni Ricci, Noemi Sofia Rabitti, Monica Laureati, Johannes Hogenboom, Luisa Pellegrino, Davide Emide, Alberto Barbiroli, and Maria Grazia Fortina. 2023. "Fine Cocoa Fermentation with Selected Lactic Acid Bacteria: Fermentation Performance and Impact on Chocolate Composition and Sensory Properties" Foods 12, no. 2: 340. https://doi.org/10.3390/foods12020340