Innovative Approaches to Fungal Food Production: Mycelial Pellet Morphology Insights
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain, Solid-State Culture, and Base Submerged Culture
2.2. Strain Identification by Internal Transcribed Spacer (ITS) Sequencing
2.3. Plackett-Burman Experimental Design
2.4. Taguchi Experimental Design
2.5. Morphological Characterization
2.6. ImageJ Software Analysis
2.7. Dry Cell Weight (DCW) and Protein Assay
2.8. Polyphenol Determination
2.9. Total Polysaccharides Assay
2.10. Statistical Analysis
3. Results and Discussions
3.1. Fungal Strain and Growth Curve
3.2. Factor Screening Using Plackett-Burman Design
3.3. Taguchi Experiment Results
3.4. Optimization of Mycelial Pellet Morphology Using Tween 80
3.5. Optimization of Mycelial Pellet Morphology through Medium Intensity
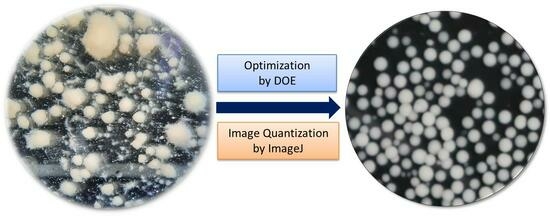
3.6. Assessment of Optimization and Food Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barzee, T.J.; Cao, L.; Pan, Z.; Zhang, R. Fungi for Future Foods. J. Future Foods 2021, 1, 25–37. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Kumar, V.; Hellwig, C.; Wikandari, R.; Harirchi, S.; Sar, T.; Taherzadeh, M.J. Filamentous Fungi for Sustainable Vegan Food Production Systems within a Circular Economy: Present Status and Future Prospects. Food Res. Int. 2022, 164, 112318. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.W.; Paul, G.C.; Thomas, C.R. Image Analysis of the Morphology of Filamentous Microorganisms. Microbiology 1998, 144, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Berovic, M.; Popovic, M. Characterization of Gas Mixed Bioreactors in Submerged Citric Acid Fermentation. Chem. Biochem. Eng. Q. 2001, 15, 65–70. [Google Scholar]
- Xia, C.; Zhang, J.; Zhang, W.; Hu, B. A New Cultivation Method for Microbial Oil Production: Cell Pelletization and Lipid Accumulation by Mucor circinelloides. Biotechnol. Biofuels 2011, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Leng, L.; Ye, C.; Lu, Q.; Addy, M.; Wang, J.; Zhou, W. A Comparative Study between Fungal Pellet- and Spore-Assisted Microalgae Harvesting Methods for Algae Bioflocculation. Bioresour. Technol. 2018, 259, 181–190. [Google Scholar] [CrossRef]
- Muradov, N.; Taha, M.; Miranda, A.F.; Wrede, D.; Kadali, K.; Gujar, A.; Mouradov, A. Fungal-Assisted Algal Flocculation: Application in Wastewater Treatment and Biofuel Production. Biotechnol. Biofuels 2015, 8, 1–23. [Google Scholar] [CrossRef]
- Bai, S.; Wang, L.; Ma, F.; Zhu, S.; Xiao, T.; Yu, T.; Wang, Y. Self-Assembly Biochar Colloids Mycelial Pellet for Heavy Metal Removal from Aqueous Solution. Chemosphere 2020, 242, 125182. [Google Scholar] [CrossRef]
- Legorreta-Castañeda, A.J.; Lucho-Constantino, C.A.; Coronel-Olivares, C.; Beltrán-Hernández, R.I.; Vázquez-Rodríguez, G.A. Biosorption of Precious Metals Present at Dilute Concentrations on Fungal Pellets. Processes 2022, 10, 645. [Google Scholar] [CrossRef]
- Dulay, R.M.R.; Ray, K.; Hou, C.T. Optimization of Liquid Culture Conditions of Philippine Wild Edible Mushrooms as a Potential Source of Bioactive Lipids. Biocatal. Agric. Biotechnol. 2015, 4, 409–415. [Google Scholar] [CrossRef]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced Materials from Fungal Mycelium: Fabrication and Tuning of Physical Properties. Sci. Rep. 2017, 7, 41292. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, Y.; Hu, W.; Shen, W.; Yu, Z.; Zhou, W.; Zhang, Y. Genetically Shaping Morphology of the Filamentous Fungus Aspergillus glaucus for Production of Antitumor Polyketide Aspergiolide A. Microb. Cell Factories 2014, 13, 20. [Google Scholar] [CrossRef]
- Cairns, T.C.; Feurstein, C.; Zheng, X.; Zheng, P.; Sun, J.; Meyer, V. A Quantitative Image Analysis Pipeline for the Characterization of Filamentous Fungal Morphologies as a Tool to Uncover Targets for Morphology Engineering: A Case Study Using aplD in Aspergillus niger. Biotechnol. Biofuels 2019, 12, 149. [Google Scholar] [CrossRef]
- Plackett, R.L.; Burman, J.P. The Design of Optimum Multifactorial Experiments. Biometrika 1946, 33, 305–325. [Google Scholar] [CrossRef]
- Abdullah, N.; Ismail, R.; Johari, N.M.K.; Annuar, M.S.M. Production of Liquid Spawn of an Edible Grey Oyster Mushroom, Pleurotus pulmonarius (Fr.) Quél by Submerged Fermentation and Sporophore Yield on Rubber Wood Sawdust. Sci. Hortic. 2013, 161, 65–69. [Google Scholar] [CrossRef]
- Wang, B.; Chen, J.; Li, H.; Sun, F.; Li, Y.; Shi, G. Pellet-Dispersion Strategy to Simplify the Seed Cultivation of Aspergillus niger and Optimize Citric Acid Production. Bioprocess Biosyst. Eng. 2017, 40, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Bakratsas, G.; Polydera, A.; Katapodis, P.; Stamatis, H. Recent Trends in Submerged Cultivation of Mushrooms and Their Application as a Source of Nutraceuticals and Food Additives. Future Foods 2021, 4, 100086. [Google Scholar] [CrossRef]
- Huang, L.; He, C.; Si, C.; Shi, H.; Duan, J. Nutritional, Bioactive, and Flavor Components of Giant Stropharia (Stropharia rugoso-annulata): A Review. J. Fungi 2023, 9, 792. [Google Scholar] [CrossRef]
- Strong, P.J.; Self, R.; Allikian, K.; Szewczyk, E.; Speight, R.; O’Hara, I.; Harrison, M.D. Filamentous Fungi for Future Functional Food and Feed. Curr. Opin. Biotechnol. 2022, 76, 102729. [Google Scholar] [CrossRef]
- Bibi, S.; Wang, Z.L.; Lin, C.; Min, S.-H.; Cheng, C.-Y. Two-Stage Cultivation Strategies for Optimal Production of Ganoderma Pellets with Potential Application in the Vegan Food Industry. JFST 2023, 60, 1793–1802. [Google Scholar] [CrossRef]
- Halprin, K.M.; Taylor, J.R.; Levine, V.; Adachi, K. A Combined Alkali Extraction-Ethidium Bromide Technique for the Measurement of DNA in Small Pieces of Tissue. J. Investig. Dermatol. 1979, 73, 359–363. [Google Scholar] [CrossRef]
- Yang, Y.; Zuzak, K.; Feng, J. An Improved Simple Method for DNA Extraction from Fungal Mycelia. Can. J. Plant Pathol. 2016, 38, 476–482. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Christiansen, J.E. Irrigation by Sprinkling. In California Agricultural Experiment Station Bulletin 670; University of California: Berkeley, CA, USA, 1942. [Google Scholar]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Supramani, S.; Jailani, N.; Ramarao, K.; Zain, N.A.M.; Klaus, A.; Ahmad, R.; Wan, W.A.A.Q.I. Pellet Diameter and Morphology of European Ganoderma pfeifferi in a Repeated-Batch Fermentation for Exopolysaccharide Production. Biocatal. Agric. Biotechnol. 2019, 19, 101118. [Google Scholar] [CrossRef]
- Handa, N. Examination on the Applicability of the Phenol Sulfuric Acid Method to the Determination of Dissolved Carbohydrate in Sea Water. J. Oceanogr. Soc. Jpn. 1966, 22, 79–86. [Google Scholar] [CrossRef]
- Olsvik, E.; Tucker, K.G.; Thomas, C.R.; Kristiansen, B. Correlation of Aspergillus niger broth rheological properties with biomass concentration and the shape of mycelial aggregates. Biotechnol. Bioeng. 1993, 42, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, J.; Mao, J.; Zhang, L.; He, C.; Chen, G.; Parkin, I.P.; Lai, Y. In Vivo and In Vitro Efficient Textile Wastewater Remediation by Aspergillus niger Biosorbent. Nanoscale Adv. 2018, 1, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Petre, M.; Teodorescu, A.; Tuluca, E.; Bejan, C.; Andronescu, A. Biotechnology of Mushroom Pellets Producing by Controlled Submerged Fermentation. Rom. Biotechnol. Lett. 2010, 15, 50–55. [Google Scholar]
- Zervakis, G.; Yiatras, P.; Balis, C. Edible Mushrooms from Olive Oil Mill Wastes. Int. Biodeterior. Biodegrad. 1996, 38, 237–243. [Google Scholar] [CrossRef]
- Wu, M.; Xu, Y.; Ding, W.; Li, Y.; Xu, H. Mycoremediation of Manganese and Phenanthrene by Pleurotus eryngii Mycelium Enhanced by Tween 80 and Saponin. Appl. Microbiol. Biotechnol. 2016, 100, 7249–7261. [Google Scholar] [CrossRef]
- Ha, H.C.; Honda, Y.; Watanabe, T.; Kuwahara, M. Production of Manganese Peroxidase by Pellet Culture of the Lignin-Degrading Basidiomycete, Pleurotus ostreatus. Appl. Microbiol. Biotechnol. 2001, 55, 704–711. [Google Scholar] [CrossRef]
- Krasnopolskaya, L.; Shuktueva, M.; Golyshkin, A.; Almyasheva, N.; Yarina, M. Optimization of the Nutrient Medium for Flammulina velutipes Submerged Biomass Production and Micromorphology of Its Mycelium. Fermentation 2021, 7, 180. [Google Scholar] [CrossRef]
- Singh, R.; Gaur, R.; Tiwari, S.; Gaur, M.K. Production of Pullulan by a Thermotolerant Aureobasidium pullulans Strain in Non-Stirred Fed Batch Fermentation Process. Braz. J. Microbiol. 2012, 43, 1042–1050. [Google Scholar] [CrossRef]
- Sornchai, A.K.P.; Chanprame, S.; Iamtham, S. Utilization of Spirulina maxima to enhance yield and cordycepin content in Cordyceps militaris artificial cultivation. J. ISSAAS 2021, 27, 1–14. [Google Scholar]
- Kirsch, L.D.S.; Macedo, A.J.P.D.; Teixeira, M.F.S. Production of Mycelial Biomass by the Amazonian Edible Mushroom Pleurotus albidus. Braz. J. Microbiol. 2016, 47, 658–664. [Google Scholar] [CrossRef]
- Papagianni, M.; Mattey, M. Morphological Development of Aspergillus niger in Submerged Citric Acid Fermentation as a Function of the Spore Inoculum Level. Application of Neural Network and Cluster Analysis for Characterization of Mycelial Morphology. Microb. Cell Factories 2006, 5, 3. [Google Scholar] [CrossRef]
- Liu, Y.S.; Wu, J.Y. Effects of Tween 80 and pH on Mycelial Pellets and Exopolysaccharide Production in Liquid Culture of a Medicinal Fungus. J. Ind. Microbiol. Biotechnol. 2012, 39, 623–628. [Google Scholar] [CrossRef]
- Luthra, U.; Chaturvedi, A.; Saini, A.S.; Bhagat, V.; Bhat, T.A.; Murukate, V.; Khandelwal, N. Study on Physiological Changes of Fungal Morphology during the Fermentation Process for the Production of Mycophenolic Acid. Am. Int. J. Contemp. Sci. Res. 2015, 2, 69–76. [Google Scholar]
- Driouch, H.; Roth, A.; Dersch, P.; Wittmann, C. Filamentous Fungi in Good Shape: Microparticles for Tailor-Made Fungal Morphology and Enhanced Enzyme Production. Bioeng. Bugs 2011, 2, 100–104. [Google Scholar] [CrossRef]
- Souza Filho, P.F.; Andersson, D.; Ferreira, J.A.; Taherzadeh, M.J. Mycoprotein: Environmental Impact and Health Aspects. World J. Microbiol. Biotechnol. 2019, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Naylor, T.W.; Williamson, T.; Trinci, P.J.; Robson, G.D.; Wiebe, M.G. Fungal Food. U.S. Patent No. 5,980,958, 9 November 1999. Available online: https://patents.google.com/patent/US5980958 (accessed on 17 September 2023).
- Trinci, A.P. The 1994 Marjory Stephenson Prize Lecture. Evolution of the Quorn Mycoprotein Fungus, Fusarium graminearum A3/5. Microbiology 1994, 140, 2181–2188. [Google Scholar] [CrossRef] [PubMed]



| Symbols | Factors | Level 1 (+1) | Level 2 (−1) | Units |
|---|---|---|---|---|
| X1 | Lignin 1 | 1 | 0 | mL |
| X2 | Inoculation | 2 | 1 | pieces |
| X3 | YE | 0.5 | 0.25 | g |
| X4 | CaCO3 | 0.5 | 0 | g |
| X5 | Olive oil | 2 | 0 | mL |
| X6 | Yolk powder | 0.5 | 0.25 | g |
| X7 | Soy powder | 0.5 | 0.25 | g |
| X8 | Tween 80 | 1 | 0 | mL |
| X9 | KH2PO4 | 2 | 1 | g |
| X10 | Mung bean powder | 0.5 | 0 | g |
| X11 | Dummy factors |
| Symbols | Factors | Level 1 | Level 2 | Level 3 | Units |
|---|---|---|---|---|---|
| A | Olive oil | 0 | 2 | 4 | mL |
| B | CaCO3 | 0 | 0.25 | 0.5 | g |
| C | YE | 0.25 | 0.50 | 0.75 | g |
| D | Soy powder | 0.00 | 0.25 | 0.50 | g |
| Trial No. | X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | X10 | X11 | DCW |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 0.5 | 0 | 0 | 0.25 | 0.5 | 1 | 2 | 0 | - | 0.50 ± 0.23 |
| 2 | 1 | 2 | 0.25 | 0.5 | 0 | 0.25 | 0.25 | 1 | 2 | 0.5 | - | 2.35 ± 0.07 |
| 3 | 0 | 2 | 0.5 | 0 | 2 | 0.25 | 0.25 | 0 | 2 | 0.5 | - | 1.64 ± 0.91 |
| 4 | 1 | 1 | 0.5 | 0.5 | 0 | 0.5 | 0.25 | 0 | 1 | 0.5 | - | 2.25 ± 0.19 |
| 5 | 1 | 2 | 0.25 | 0.5 | 2 | 0.25 | 0.5 | 0 | 1 | 0 | - | 2.48 ± 0.31 |
| 6 | 1 | 2 | 0.5 | 0 | 2 | 0.5 | 0.25 | 1 | 1 | 0 | - | 2.81 ± 0.18 |
| 7 | 0 | 2 | 0.5 | 0.5 | 0 | 0.5 | 0.5 | 0 | 2 | 0 | - | 1.19 ± 1.01 |
| 8 | 0 | 1 | 0.5 | 0.5 | 2 | 0.25 | 0.5 | 1 | 1 | 0.5 | - | 4.12 ± 0.30 |
| 9 | 0 | 1 | 0.25 | 0.5 | 2 | 0.5 | 0.25 | 1 | 2 | 0 | - | 3.73 ± 1.28 |
| 10 | 1 | 1 | 0.25 | 0 | 2 | 0.5 | 0.5 | 0 | 2 | 0.5 | - | 0.58 ± 0.30 |
| 11 | 0 | 2 | 0.25 | 0 | 0 | 0.5 | 0.5 | 1 | 1 | 0.5 | - | 1.77 ± 0.38 |
| 12 | 0 | 1 | 0.25 | 0 | 0 | 0.25 | 0.25 | 0 | 1 | 0 | - | 0.94 ± 0.19 |
| Effect 1 | −0.401 | 0.021 | 0.111 | 1.313 | 1.06 | 0.049 | −0.514 | 1.031 | −0.729 | 0.176 | 0.062 | |
| SS 2 | 0.483 | 0.001 | 0.037 | 5.173 | 3.37 | 0.007 | 0.791 | 3.19 | 1.593 | 0.093 | 0.012 | |
| MS 3, 4 | 0.483 | 0.001 | 0.037 | 5.173 | 3.37 | 0.007 | 0.791 | 3.19 | 1.593 | 0.093 | 0.012 | |
| F-value 5 | 41.95 | 0.11 | 3.2 | 449.6 | 292.9 | 0.63 | 68.78 | 277.3 | 138.5 | 8.11 | 1 | |
| P 6 | 0.098 | 0.793 | 0.325 | * 0.030 | * 0.037 | 0.573 | 0.076 | * 0.038 | 0.054 | 0.215 | 0.5 |
| Factors | Olive oil | CaCO3 | YE | Soy Powder | Biomass | S/N Ratio | |
|---|---|---|---|---|---|---|---|
| Trial no. | L1 | 0.0 | 0.0 | 0.3 | 0.0 | 0.65 ± 0.16 | −4.52 |
| L2 | 0.0 | 0.3 | 0.5 | 0.3 | 1.36 ± 0.09 | 2.61 | |
| L3 | 0.0 | 0.5 | 0.8 | 0.5 | 1.71 ± 0.22 | 4.47 | |
| L4 | 2.0 | 0.0 | 0.5 | 0.5 | 1.61 ± 0.28 | 3.75 | |
| L5 | 2.0 | 0.3 | 0.8 | 0.0 | 2.11 ± 0.37 | 6.03 | |
| L6 | 2.0 | 0.5 | 0.3 | 0.3 | 1.66 ± 0.14 | 4.29 | |
| L7 | 4.0 | 0.0 | 0.8 | 0.3 | 1.27 ± 0.21 | 1.68 | |
| L8 | 4.0 | 0.3 | 0.3 | 0.5 | 1.52 ± 0.31 | 3.03 | |
| L9 | 4.0 | 0.5 | 0.5 | 0.0 | 1.81 ± 0.29 | 4.78 | |
| S/N ratio | Level 1 | 0.85 | 0.30 | 0.93 | 2.10 | ||
| Level 2 | 4.69 | 3.89 | 3.71 | 2.86 | |||
| Level 3 | 3.16 | 4.51 | 4.06 | 3.75 | |||
| SS 1 | 22.38 | 30.97 | 17.63 | 4.09 | |||
| Contribution 2 | 30% | 41% | 23% | 5% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.-Y.; Wang, Y.-S.; Wang, Z.-L.; Bibi, S. Innovative Approaches to Fungal Food Production: Mycelial Pellet Morphology Insights. Foods 2023, 12, 3477. https://doi.org/10.3390/foods12183477
Cheng C-Y, Wang Y-S, Wang Z-L, Bibi S. Innovative Approaches to Fungal Food Production: Mycelial Pellet Morphology Insights. Foods. 2023; 12(18):3477. https://doi.org/10.3390/foods12183477
Chicago/Turabian StyleCheng, Chih-Yu, Yu-Sheng Wang, Zhong-Liang Wang, and Sidra Bibi. 2023. "Innovative Approaches to Fungal Food Production: Mycelial Pellet Morphology Insights" Foods 12, no. 18: 3477. https://doi.org/10.3390/foods12183477