Cashew By-Product as a Functional Substrate for the Development of Probiotic Fermented Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cashew By-Product (CB)
2.2. Fermentability Assay of Probiotic and Starter Bacteria in CB
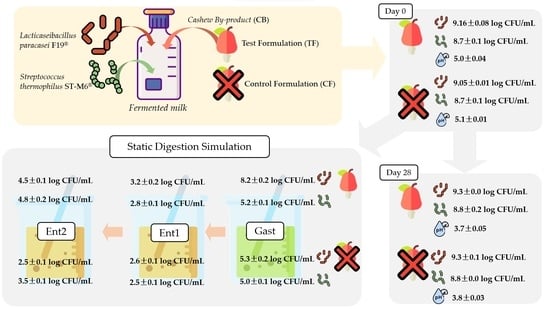
2.3. Cultures Employed and Fermented Milk Production
2.4. Evaluation of Microorganism Survival during Storage
2.5. Physicochemical Characterization
2.6. Evaluation of Fatty Acid Profile
2.7. Viability of Microorganisms under In Vitro-Simulated Gastrointestinal Conditions
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical and Functional Characteristics of the Cashew By-Product (CB)
3.2. Probiotic Selection
3.3. Viability of Microorganisms Used in Fermented Milk during Storage, pH, and Titratable Acidity
3.4. Physical–Chemical Composition of Fermented Milk
3.5. Survival of Microorganisms in Fermented Milk under In Vitro-Simulated Gastrointestinal Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliveira, N.N.; Mothé, C.G.; Mothé, M.G.; de Oliveira, L.G. Cashew nut and cashew apple: A scientific and technological monitoring worldwide review. J. Food Sci. Technol. 2020, 57, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Dheeraj; Srivastava, A.; Mishra, A. Mitigation of cashew apple fruits astringency. Env. Sustain. 2023, 31, 1–11. [Google Scholar] [CrossRef]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. What’s new in biopotential of fruit and vegetable by-products applied in the food processing industry. Trends Food Sci. Technol. 2017, 67, 150–159. [Google Scholar] [CrossRef]
- De Souza, C.B.; Jonathan, M.; Saad, S.M.I.; Schols, H.A.; Venema, K. Degradation of fibres from fruit by-products allows selective modulation of the gut bacteria in an in vitro model of the proximal colon. J. Funct. Foods 2019, 57, 275–285. [Google Scholar] [CrossRef]
- Medeiros, I.U.D.; Aquino, J.S.; Cavalcanti, N.S.H.; Campos, A.R.N.; Cordeiro, A.M.T.M.; Damasceno, K.S.F.S.C.; Hoskin, R.T. Characterization and functionality of fibre-rich pomaces from the tropical fruit pulp industry. Br. Food J. 2019, 122, 813–826. [Google Scholar] [CrossRef]
- Zhang, L.; Carmody, R.N.; Kalariya, H.M.; Duran, R.M.; Moskal, K.; Poulev, A.; Roopchand, D.E.; Kuhn, P.; Tveter, K.M.; Turnbaugh, P.J.; et al. Grape proanthocyanidin-induced intestinal bloom of Akkermansia muciniphila is dependent on its baseline abundance and precedes activation of host genes related to metabolic health. J. Nutr. Biochem. 2018, 56, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Villacís-Chiriboga, J.; Elst, K.; Van Camp, J.; Vera, E.; Ruales, J. Valorization of byproducts from tropical fruits: Extraction methodologies, applications, environmental, and economic assessment: A review (Part 1: General overview of the byproducts, traditional biorefinery practices, and possible applications). Compr. Rev. Food Sci. Food Saf. 2020, 19, 405–447. [Google Scholar] [CrossRef]
- Sayago-Ayerdi, S.; García-Martínez, D.L.; Ramírez-Castillo, A.C.; Ramírez-Concepción, H.R.; Viuda-Martos, M. Tropical fruits and their co-products as bioactive compounds and their health effects: A review. Foods 2021, 10, 1952. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Bernatoniene, J. Antioxidant effects of Schisandra chinensis fruits and their active constituents. Antioxidants 2021, 10, 620. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.S. Bioactive compounds of citrus fruits: A review of composition and health benefits of carotenoids, flavonoids, limonoids, and terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Kowalska, K. Lingonberry (Vaccinium vitisidaea L.) fruit as a source of bioactive compounds with health-promoting effects—A review. Int. J. Mol. Sci. 2021, 22, 5126. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Alqarni, M.H.; Alsayari, A.; Foudah, A.I.; Aljarba, T.M.; Mukim, M.; Alamri, M.A.; Abullais, S.S.; Wahab, S. Anti-diabetic activity of bioactive compound extracted from spondias mangifera fruit: In-vitro and molecular docking approaches. Plants 2022, 11, 562. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Albuquerque, M.A.; Bedani, R.; Vieira, A.D.; LeBlanc, J.G.; Saad, S.M.I. Supplementation with fruit and okara soybean by-products and amaranth flour increases the folate production by starter and probiotic cultures. Int. J. Food Microbiol. 2016, 236, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.D.S.; Bedani, R.; Albuquerque, M.A.C.; Biscola, V.; Saad, S.M.I. The impact of fruit and soybean by-products and amaranth on the growth of probiotic and starter microorganisms. Food Res. Int. 2017, 97, 356–363. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Ballan, R.; Battistini, C.; Xavier-Santos, D.; Saad, S.M.I. Interactions of probiotics and prebiotics with the gut microbiota. Prog. Mol. Biol. Transl. Sci. 2020, 171, 265–300. [Google Scholar] [CrossRef]
- Gómez-García, R.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Valorization of melon fruit (Cucumis melo L.) by-products: Phytochemical and Biofunctional properties with Emphasis on Recent Trends and Advances. Trends Food Sci. Technol. 2020, 99, 507–519. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Microbial production of lactic acid: The latest development. Crit. Rev. Biotechnol. 2016, 36, 967–977. [Google Scholar] [CrossRef]
- Praia, A.B.; Herkenhoff, M.E.; Broedel, O.; Frohme, M.; Saad, S.M.I. Sour Beer with Lacticaseibacillus paracasei subsp. paracasei F19: Feasibility and Influence of Supplementation with Spondias mombin L. Juice and/or By-Product. Foods 2022, 11, 4068. [Google Scholar] [CrossRef]
- Battistini, C.; Herkenhoff, M.E.; de Souza Leite, M.; Vieira, A.D.S.; Bedani, R.; Saad, S.M.I. Brewer’s Spent Grain Enhanced the Recovery of Potential Probiotic Strains in Fermented Milk After Exposure to In Vitro-Simulated Gastrointestinal Conditions. Probiotics Antimicrob. Proteins 2023, 15, 326–337. [Google Scholar] [CrossRef]
- Herkenhoff, M.E.; Battistini, C.; Praia, A.B.; Rossini, B.C.; Dos Santos, L.D.; Brödel, O.; Frohme, M.; Saad, S.M.I. The combination of omics strategies to evaluate starter and probiotic strains in the Catharina sour Brazilian-style beer. Food Res. Int. 2023, 167, 112704. [Google Scholar] [CrossRef]
- Shahrokhi, M.; Nagalli, S. Probiotics StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Albuquerque, M.A.C.; Bedani, R.; LeBlanc, J.G.; Saad, S.M.I. Passion fruit by-product and fructooligosaccharides stimulate the growth and folate production by starter and probiotic cultures in fermented soymilk. Int. J. Food Microbiol. 2017, 261, 35–41. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Saldivar, S.O.S. Cereals: Dietary Importance. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 703–711. [Google Scholar] [CrossRef]
- Kamal-Eldin, A. Dietary Fiber: Bran. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 378–382. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Armada, R.D.P.; Pérez-Cózar, M.L.; Rupérez, P.; Redondo-Cuenca, A.; Villanueva-Suárez, M.J. Apple by-product dietary fibre exhibits potential prebiotic and hypolipidemic effectsin high-fat fed Wistar rats. Bioact. Carbohydr. Diet. Fibre 2019, 23, 100219. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Blancas-Benítez, F.J.; Sáyago-Ayerdi, S.G. Polyphenols associated with dietary fibers in plant foods: Molecular interactions and bioaccessibility. Curr. Opin. Food Sci. 2017, 13, 84–88. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P. Non-covalent dietary fiber—Polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]
- Koutsos, A.; Lima, M.; Conterno, L.; Gasperotti, M.; Bianchi, M.; Fava, F.; Vrhovsek, U.; Lovegrove, J.A.; Tuohy, K.M. Effects of commercial apple varieties on human gut microbiota composition and metabolic output using an in vitro colonic model. Nutrients 2017, 9, 533. [Google Scholar] [CrossRef]
- Chacar, S.; Itani, T.; Hajal, J.; Saliba, Y.; Louka, N.; Faivre, J.F.; Maroun, R.; Fares, N. The Impact of Long-Term Intake of Phenolic Compounds-Rich Grape Pomace on Rat Gut Microbiota. J. Food Sci. 2018, 83, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Carranza, P.; Ávila-Sosa, R.; Guerrero-Beltrán, J.A.; Navarro-Cruz, A.R.; Corona-Jiménez, E.; Ochoa-Velasco, C.E. Optimization of Antioxidant Compounds Extraction from Fruit By-Products: Apple Pomace, Orange and Banana Peel. J. Food Process. Preserv. 2016, 40, 103–115. [Google Scholar] [CrossRef]
- Batista, K.S.; Alves, A.F.; Lima, M.D.S.; da Silva, L.A.; Lins, P.P.; de Sousa Gomes, J.A.; Silva, A.S.; Toscano, L.T.; Meireles, B.R.L.d.A.; Cordeiro, A.M.T.d.M.; et al. Beneficial effects of consumption of acerola, cashew or guava processing by-products on intestinal health and lipid metabolism in dyslipidaemic female Wistar rats. Br. J. Nutr. 2018, 119, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.C.Q.; Madruga, M.S.; Pintado, M.M.E.; Almeida, G.H.O.; Alves, A.P.V.; Dantas, F.A.; Bezerra, J.K.G.; de Melo, M.F.F.T.; Viera, V.B.; Soares, J.K.B. Cashew nuts (Anacardium occidentale L.) decrease visceral fat, yet augment glucose in dyslipidemic rats. PLoS ONE 2019, 14, e0225736. [Google Scholar] [CrossRef] [PubMed]
- Multari, S.; Marsol-Vall, A.; Heponiemi, P.; Suomela, J.P.; Yang, B. Changes in the volatile profile, fatty acid composition and other markers of lipid oxidation of six different vegetable oils during short-term deep-frying. Food Res. Int. 2019, 122, 318–329. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Xia, Q.; Lin, L. Analysis of Physicochemical Properties, Lipid Composition, and Oxidative Stability of Cashew Nut Kernel Oil. Foods 2023, 12, 693. [Google Scholar] [CrossRef]
- Lee, H.; Li, Z.; Christensen, B.; Peng, Y.; Li, X.; Hernell, O.; Lönnerdal, B.; Slupsky, C.M. Metabolic Phenotype and Microbiome of Infants Fed Formula Containing Lactobacillus paracasei Strain F-19. Front. Pediatr. 2022, 10, 856951. [Google Scholar] [CrossRef]
- Jones, R.M. The use of Lactobacillus casei and Lactobacillus paracasei in clinical trials for the improvement of human health. In The Microbiota in Gastrointestinal Pathophysiology: Implications for Human Health, Prebiotics, Probiotics, and Dysbiosis; Elsevier: Amsterdam, The Netherlands, 2017; pp. 99–108. [Google Scholar]
- Gu, Y.; Li, X.; Chen, H.; Guan, K.; Qi, X.; Yang, L.; Ma, Y. Evaluation of FAAs and FFAs in yogurts fermented with different starter cultures during storage. J. Food Compos. Anal. 2021, 96, 103666. [Google Scholar] [CrossRef]
- Vieira, A.D.S.; Battistini, C.; Bedani, R.; Saad, S.M.I. Acerola by-product may improve the in vitro gastrointestinal resistance of probiotic strains in a plant-based fermented beverage. LWT Food Sci. Technol. 2021, 141, 110858. [Google Scholar] [CrossRef]
- Health Canada. Probiotic Claims. 2019. Available online: https://inspection.canada.ca/eng/1297964599443/1297965645317 (accessed on 4 August 2023).
- FAO. Statistical database from crop production (FAOSTAT). Available online: http://www.fao.org/faostat/en/#data/QC/ (accessed on 24 August 2023).
- Vieira, A.D.S.; de Souza, C.B.; Padilha, M.; Zoetendal, E.G.; Smidt, H.; Saad, S.M.I.; Venema, K. Impact of a fermented soy beverage supplemented with acerola by-product on the gut microbiota from lean and obese subjects using an in vitro model of the human colon. Appl. Microbiol. Biotechnol. 2021, 105, 3771–3785. [Google Scholar] [CrossRef]
- Karaca, O.B.; Güzeler, N.; Tangüler, H.; Yasar, K.; Akın, M.B. Effects of Apricot Fibre on the Physicochemical Characteristics, the Sensory Properties and Bacterial Viability of Nonfat Probiotic Yoghurts. Foods 2019, 8, 33. [Google Scholar] [CrossRef]
- Churakov, M.; Karlsson, J.; Rasmussen, A.E.; Holtenius, K. Milk fatty acids as indicators of negative energy balance of dairy cows in early lactation. Animal 2021, 15, 100253. [Google Scholar] [CrossRef]
- Unger, A.L.; Torres-Gonzalez, M.; Kraft, J. Dairy Fat Consumption and the Risk of Metabolic Syndrome: An Examination of the Saturated Fatty Acids in Dairy. Nutrients 2019, 11, 2200. [Google Scholar] [CrossRef] [PubMed]
- Gholami, Z.; Khosravi-Darani, K. An Overview of Conjugated Linoleic Acid: Microbial Production and Application. Mini-Rev. Med. Chem. 2014, 14, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Marand, M.A.; Amjabi, S.; Roufegarinejad, L.; Jafari, S.M. Fortification of yogurt with flaxseed powder and evaluation of its fatty acid profile, physicochemical, antioxidant, and sensory properties. Powder Technol. 2020, 359, 76–84. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Mehta, T.; Pastoriza, S.; Kramer, D.L.; Paliy, O.; Rufián-Henares, J.A. Potential probiotic salami with dietary fiber modulates antioxidant capacity, short chain fatty acid production and gut microbiota community structure. LWT Food Sci. Technol. 2019, 105, 355–362. [Google Scholar] [CrossRef]
- Zhang, S.; Willett, S.A.; Hyatt, J.R.; Martini, S.; Akoh, C.C. Phenolic compounds as antioxidants to improve oxidative stability of menhaden oil-based structured lipid as butterfat analog. Food Chem. 2021, 334, 127584. [Google Scholar] [CrossRef] [PubMed]
- Menezes, F.N.D.D.; da Cruz Almeida, E.T.; da Silva Vieira, A.R.; de Souza Aquino, J.; Dos Santos Lima, M.; Magnani, M.; de Souza, E.L. Impact of Cashew (Anacardium occidentale L.) by-Product on Composition and Metabolic Activity of Human Colonic Microbiota In Vitro Indicates Prebiotic Properties. Curr. Microbiol. 2021, 78, 2264–2274. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Sauceda, A.E.; Palafox-Carlos, H.; Sáyago-Ayerdi, S.G.; Ayala-Zavala, J.F.; Bello-Perez, L.A.; Alvarez-Parrilla, E.; de la Rosa, L.A.; González-Córdova, A.F.; González-Aguilar, G.A. Dietary fiber and phenolic compounds as functional ingredients: Interaction and possible effect after ingestion. Food Funct. 2014, 5, 1063–1072. [Google Scholar] [CrossRef]
- Beukema, M.; Faas, M.M.; De Vos, P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef]
- Larsen, N.; Cahú, T.B.; Saad, S.M.I.; Blennow, A.; Jespersen, L. The effect of pectins on survival of probiotic Lactobacillus spp. in gastrointestinal juices is related to their structure and physical properties. Food Microbiol. 2018, 74, 11–20. [Google Scholar] [CrossRef]
- Larsen, N.; de Souza, C.B.; Krych, L.; Kot, W.; Leser, T.D.; Sørensen, O.B.; Blennow, A.; Venema, K.; Jespersen, L. Effect of potato fiber on survival of Lactobacillus species at simulated gastric conditions and composition of the gut microbiota in vitro. Food Res. Int. 2019, 125, 108644. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, Y.; Li, J.; Ban, L.; Yang, L.; Wang, S.; Zhu, L.; Song, H.; Liu, H. The adhesion of the gut microbiota to insoluble dietary fiber from soy hulls promoted the proliferation of probiotics in vitro. LWT 2022, 153, 112560. [Google Scholar] [CrossRef]


| Strain | Broth | Agar | Incubation (at 37 °C) |
|---|---|---|---|
| Streptococcus thermophilus TH-4® a | HJ 1 | M17 2 | Aerobiosis |
| Streptococcus. thermophilus ST-M6® a | |||
| Streptococcus thermophilus TA-40® b | |||
| Lactobacillus acidophilus LA-5® c | MRS 3 | MRS maltose 4 | Aerobiosis |
| Limosilactobacillus fermentum PCC® c | MRS 3 | MRS 5 | Anaerobiosis 7 |
| Limosilactobacillus reuteri RC-14® c | MRS 3 | Acidified MRS 6 | Anaerobiosis 7 |
| Lacticaseibacillus. paracasei subsp. paracasei 431® c | |||
| Lacticaseibacillus. paracasei subsp. paracasei F-19® c | |||
| Lacticaseibacillus. rhamnosus GR-1® c | MRS 3 | Acidified MRS 6 | Aerobiosis |
| Lacticaseibacillus. rhamnosus LGG® c | |||
| Bifidobacterium animalis subsp. lactis BB-12® c | MRS cysteine 8 | LP-MRS 9 | Anaerobiosis 7 |
| Bifidobacterium. longum BB-46® c | |||
| Bifidobacterium longum subsp. infantis BB-02® c |
| Strains | Δ48 | Δ24 | ||
|---|---|---|---|---|
| MRSm + CB | MRSm Control | MRSm + CB | MRSm Control | |
| L. reuteri RC-14 | 3.32 ± 0.13 aA | 2.57 ± 0.13 B | 3.64 ± 0.07 aA | 3.27 ± 0.02 B |
| L. paracasei subsp. paracasei 431 | 2.92 ± 0.17 abA | 2.79 ± 0.06 A | - | - |
| L. acidophilus LA-5 | 2.87 ± 0.09 abB | 3.14 ± 0.02 A | - | - |
| L. paracasei subsp. paracasei F19 | 2.80 ± 0.10 abA | 2.35 ± 0.03 B | 3.36 ± 0.22 abA | 2.55 ± 0.08 B |
| B. animalis subsp. lactis BB-12 | 2.79 ± 0.33 abA | 2.62 ±0.06 A | - | - |
| L. rhamnosus GR-1 | 2.56 ± 0.08 bc | - | - | - |
| L. rhamnosus LGG | 2.56 ± 0.13 bc | - | - | - |
| B. longum subsp. infantis BB-02 | 2.28 ± 0.19 bc | - | - | - |
| L. fermentum PCC | 2.23 ± 0.10 bc | - | - | - |
| B. longum BB-46 | 2.02 ± 0.16 c | - | - | - |
| S. thermophilus ST-M6 | 3.74 ± 0.12 A | −0.21 ± 0.15 B | 0.85 ± 0.13 A | 0.11 ± 0.04 B |
| S. thermophilus TA-40 | −1.56 ± 0.11 A | −2.19 ± 0.10 B | −0.32 ± 0.04 A | −0.76 ± 0.08 B |
| S. thermophilus TH-04 | −0.32 (−0.52–−0.02) A | −2.20 (−2.43–−2.11) B | −0.52 ± 0.03 A | −0.71 ± 0.17 A |
| Ingredients | Formulations (g/100 mL of Milk) | |
|---|---|---|
| Test (TF) | Control (CT) | |
| Dehydrated cashew by-product (CB) | 2.5 | - |
| Skimmed milk powder without lactose (Ninho®, Nestlé, Araras, Brazil) | 2.5 | 2.5 |
| Sucrose (União®, Piracicaba, Brazil) | 5.0 | 5.0 |
| Unflavored gelatin (Modelez®, Curitiba, Brazil) | 0.1 | 0.1 |
| Parameter or Composite | CB | CF | TF |
|---|---|---|---|
| Humidity g/100 g | 2.85 ± 0.11 | 80.57 ± 0.15 * | 78.36 ± 0.27 |
| Ashes g/100 g | 1.25 ± 0.02 | 0.79 ± 0.02 | 0.83 ± 0.01 * |
| Proteins g/100 g | 10.67 ± 0.02 | 3.69 ± 0.03 | 3.74 ± 0.30 |
| Carbohydrates g/100 g | 9.52 ± 0.13 | 10.89 ± 0.15 | 11.18 ± 0.28 |
| Total fat g/100 g | 4.47 ± 0.11 | 4.06 ± 0.03 | 4.11 ± 0.23 |
| Palmitic acid (C16:0) µg/g fat | 867.6 | 1449.0 ± 177 | 1341.0 ± 85.0 |
| Palmitoleic acid (C16:1) µg/g fat | 43.8 | 45.8 ± 8.1 | 43.8 ± 3.3 |
| Stearic acid (C18:0) µg/ g fat | 350.2 | 691.1 ± 75.3 | 620.0 ± 38.0 |
| Oleic acid (C18:1 ɷ-9) µg/g fat | 1336.6 | 643.0 ± 109 | 708.3 ± 61.0 |
| Linoleic acid (C18:2 ɷ-6) µg/g fat | 90.2 | 77.0 ± 13.9 | 77.0 ± 6.3 |
| Linolenic acid (C18:3 ɷ-3) µg/g fat | 54.5 | - | 8.6 ± 0.9 |
| cis-13-eicosanoic acid µg/g fat | 33.4 | - | - |
| Rumenic Acid (C18:2) µg/g fat | - | 8.4 ± 1.5 | 7.8 ± 1.0 |
| Strain | Period | Formulation | F0 | Gast | Ent1 | Ent2 |
|---|---|---|---|---|---|---|
| F19 | D7 | CF | 9.05 ± 0.01 aB | 5.28 ± 0.23 bD | 2.55 ± 0.06 cB | 2.53± 0.08 cC |
| TF | 9.16 ± 0.08 aAB | 8.21 ± 0.17 bB | 3.15 ± 0.24 dAB | 4.52 ± 0.05 cB | ||
| D14 | CF | 9.18 ± 0.01 aAB | 5.20 ± 0.16 bD | 3.67 ± 0.11 cA | 3.93 ± 0.16 cB | |
| TF | 9.42 ± 0.09 aA | 9.26 ± 0.01 aA | 3.66 ± 0.08 dA | 4.43 ± 0.11 cB | ||
| D28 | CF | 9.26 ± 0.01 aAB | 4.81 ± 0.09 bD | 3.26 ± 0.11 dA | 4.19 ± 0.13 cB | |
| TF | 9.23 ± 0.07 aAB | 6.55 ± 0.05 bC | 3.59 ± 0.06 dA | 4.65 ± 0.17 cA | ||
| ST-M6 | D7 | CF | 8.70 ± 0.09 aA | 5.01 ± 0.08 bA | 2.54 ± 0.11 dB | 3.55 ± 0.04 cB |
| TF | 8.81 ± 0.08 aA | 5.14 ± 0.07 bA | 2.75 ± 0.34 cAB | 4.73 ± 0.25 bA | ||
| D14 | CF | 9.06 ± 0.11 aA | 5.07 ± 0.15 bA | 3.73 ± 0.08 cA | 3.78 ± 0.06 cAB | |
| TF | 8.85 ± 0.22 aA | 2.97 ± 0.20 cB | 2.52 ± 0.18 cB | 4.29 ± 0.10 bA | ||
| D28 | CF | 8.58 ± 0.31 aA | 5.11 ± 0.21 bA | 3.58 ± 0.17 cA | 4.19 ± 0.16 cAB | |
| TF | 8.52 ± 0.28 aA | 5.20 ± 0.16 bA | 3.50 ± 0.06 dA | 4.49 ± 0.13 cA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herkenhoff, M.E.; de Medeiros, I.U.D.; Garutti, L.H.G.; Salgaço, M.K.; Sivieri, K.; Saad, S.M.I. Cashew By-Product as a Functional Substrate for the Development of Probiotic Fermented Milk. Foods 2023, 12, 3383. https://doi.org/10.3390/foods12183383
Herkenhoff ME, de Medeiros IUD, Garutti LHG, Salgaço MK, Sivieri K, Saad SMI. Cashew By-Product as a Functional Substrate for the Development of Probiotic Fermented Milk. Foods. 2023; 12(18):3383. https://doi.org/10.3390/foods12183383
Chicago/Turabian StyleHerkenhoff, Marcos Edgar, Igor Ucella Dantas de Medeiros, Luiz Henrique Grotto Garutti, Mateus Kawata Salgaço, Katia Sivieri, and Susana Marta Isay Saad. 2023. "Cashew By-Product as a Functional Substrate for the Development of Probiotic Fermented Milk" Foods 12, no. 18: 3383. https://doi.org/10.3390/foods12183383