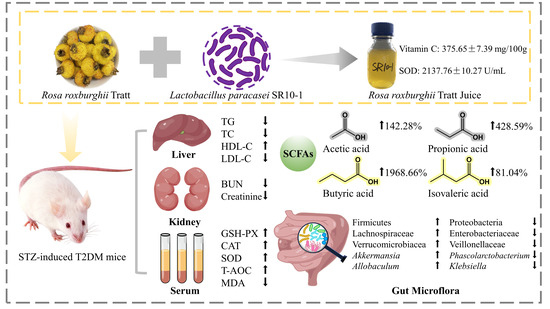
Effect and Correlation of Rosa roxburghii Tratt Juice Fermented by Lactobacillus paracasei SR10-1 on Oxidative Stress and Gut Microflora Dysbiosis in Streptozotocin (STZ)-Induced Type 2 Diabetes Mellitus Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. FRRT Preparation
2.2. Animal Experiment
2.3. Sample Collection
2.4. Oral Glucose Tolerance Test
2.5. Organ Coefficient
2.6. Biochemical Analysis of Liver and Kidney
2.7. Histopathological Analysis
2.8. Determination of Antioxidant Enzyme Activities
2.9. Analysis of Short-Chain Fatty Acids
2.10. Analysis of Gut Microbiota
2.11. Statistical Analysis
3. Results
3.1. Body Weight and Fasting Blood Glucose
3.2. Oral Glucose Tolerance
3.3. Effects of FRRT on Liver and Kidney
3.4. Histological Traits of Liver and Kidney
3.5. Effects of FRRT on Oxidative Stress
3.6. Short-Chain Fatty Acids
3.7. Composition Analysis of the Gut Microbiota
3.8. Correlation between Oxidative Stress and Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 Diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 Diabetes Mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef]
- Laakso, M. Biomarkers for Type 2 Diabetes. Mol. Metab. 2019, 27, S139–S146. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2020, 19, 55–71. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef]
- Bordalo Tonucci, L.; Dos Santos, K.M.O.; De Luces Fortes Ferreira, C.L.; Ribeiro, S.M.R.; De Oliveira, L.L.; Martino, H.S.D. Gut Microbiota and Probiotics: Focus on Diabetes Mellitus. Crit. Rev. Food Sci. Nutr. 2015, 57, 2296–2309. [Google Scholar] [CrossRef] [PubMed]
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2021, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Ma, L.; Xu, Y.; Wu, J.; Yu, Y.; Peng, J.; Tang, D.; Zou, B.; Li, L. Effects of Probiotic Litchi Juice on Immunomodulatory Function and Gut Microbiota in Mice. Food Res. Int. 2020, 137, 109433. [Google Scholar] [CrossRef]
- Hu, R.; Zeng, F.; Wu, L.; Wan, X.; Chen, Y.; Zhang, J.; Liu, B. Fermented Carrot Juice Attenuates Type 2 Diabetes by Mediating Gut Microbiota in Rats. Food Funct. 2019, 10, 2935–2946. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wen, J.-J.; Hu, J.-L.; Nie, Q.-X.; Chen, H.-H.; Xiong, T.; Nie, S.-P.; Xie, M.-Y. Fermented Momordica charantia L. Juice Modulates Hyperglycemia, Lipid Profile, and Gut Microbiota in Type 2 Diabetic Rats. Food Res. Int. 2019, 121, 367–378. [Google Scholar] [CrossRef]
- Zhong, H.; Abdullah; Deng, L.; Zhao, M.; Tang, J.; Liu, T.; Zhang, H.; Feng, F. Probiotic-Fermented Blueberry Juice Prevents Obesity and Hyperglycemia in High Fat Diet-Fed Mice in Association with Modulating the Gut Microbiota. Food Funct. 2020, 11, 9192–9207. [Google Scholar] [CrossRef]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and Management of Type 2 Diabetes: Dietary Components and Nutritional Strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-T.; Lv, M.-J.; An, J.-Y.; Fan, X.-H.; Dong, M.-Z.; Zhang, S.-D.; Wang, J.-D.; Wang, Y.-Q.; Cai, Z.-H.; Fu, Y.-J. Botanical Characteristics, Phytochemistry and Related Biological Activities of Rosa roxburghii Tratt Fruit, and Its Potential Use in Functional Foods: A Review. Food Funct. 2021, 12, 1432–1451. [Google Scholar] [CrossRef]
- Su, J.; Fu, X.; Huang, Q.; Liu, G.; Li, C. Phytochemical Profile, Bioactivity and Prebiotic Potential of Bound Polyphenols Released from Rosa roxburghii Fruit Pomace Dietary Fiber during in vitro Digestion and Fermentation. Food Funct. 2022, 13, 8880–8891. [Google Scholar] [CrossRef]
- Wang, R.; He, R.; Li, Z.; Lin, X.; Wang, L. HPLC-Q-Orbitrap-MS/MS Phenolic Profiles and Biological Activities of Extracts from Roxburgh Rose (Rosa roxburghii Tratt.) Leaves. Arab. J. Chem. 2021, 14, 103257. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, P.; Li, C.; Xu, F.; Chen, J. A Polysaccharide from Rosa roxburghii Tratt Fruit Attenuates High-Fat Diet-Induced Intestinal Barrier Dysfunction and Inflammation in Mice by Modulating the Gut Microbiota. Food Funct. 2021, 13, 530–547. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Polysaccharide from Rosa roxburghii Tratt Fruit Attenuates Hyperglycemia and Hyperlipidemia and Regulates Colon Microbiota in Diabetic Db/Db Mice. J. Agric. Food Chem. 2019, 68, 147–159. [Google Scholar] [CrossRef]
- Xu, S.-J.; Zhang, F.; Wang, L.-J.; Hao, M.-H.; Yang, X.; Li, N.; Ji, H.; Xu, P. Flavonoids of Rosa roxburghii Tratt Offers Protection against Radiation Induced Apoptosis and Inflammation in Mouse Thymus. Apoptosis 2018, 23, 470–483. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Y.; Lu, M.; Yu, L.; Tan, S.; Ren, T. Effects of Rosa roxburghii Tratt Glycosides and Quercetin on D-galactose-induced Aging Mice Model. J. Food Biochem. 2022, 46, e14425. [Google Scholar] [CrossRef]
- Xu, J.; Vidyarthi, S.K.; Bai, W.; Pan, Z. Nutritional Constituents, Health Benefits and Processing of Rosa roxburghii: A Review. J. Funct. Foods 2019, 60, 103456. [Google Scholar] [CrossRef]
- Chen, C.; Tan, S.; Ren, T.; Wang, H.; Dai, X.; Wang, H. Polyphenol from Rosa roxburghii Tratt Fruit Ameliorates the Symptoms of Diabetes by Activating the P13K/AKT Insulin Pathway in Db/Db Mice. Foods 2022, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Z.; Wang, M.; Long, M.; Ren, T.; Chen, C.; Dai, X.; Yang, S.; Tan, S. The Effect of Polyphenol Extract from Rosa roxburghii Fruit on Plasma Metabolome and Gut Microbiota in Type 2 Diabetic Mice. Foods 2022, 11, 1747. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, S.; Yuan, M.; Zhang, M.; Tang, L.; Wang, P.; Liu, Y.; Xu, C.; Luo, P.; Gao, X. Fermented Rosa roxburghii Tratt Juice Alleviates High-Fat Diet-Induced Hyperlipidemia in Rats by Modulating Gut Microbiota and Metabolites. Front. Pharmacol. 2022, 13, 883629. [Google Scholar] [CrossRef]
- Feng, D.; Hu, P.; Xu, H.; Zhang, J.; Shi, Y.; Wu, W.; Li, J.; Zuo, Y.; Li, J. Hypoglycemic and Hypolipidemic Activities of Rosa roxburghii Juice Fermented by Lactic Acid Bacteria in vitro. Food Ferment. Ind. 2022, 48, 212–219. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Zhang, Y.; Shi, Y.; Feng, D.; Zuo, Y.; Hu, P. Effect and Correlation of Rosa roxburghii Tratt Fruit Vinegar on Obesity, Dyslipidemia and Intestinal Microbiota Disorder in High-Fat Diet Mice. Foods 2022, 11, 4108. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, H.; Xu, K.; Du, Y.; Liu, J.; Wang, J.; Jiang, Y. Fecal Metabolomics Reveals the Positive Effect of Ethanol Extract of Propolis on T2DM Mice. Food Sci. Hum. Wellness 2022, 12, 161–172. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Guo, M. Autogenic Successions of Bacteria and Fungi in Kefir Grains from Different Origins When Sub-Cultured in Goat Milk. Food Res. Int. 2020, 138, 109784. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Yang, K.; Bai, Y.; Yu, N.; Lu, B.; Han, G.; Yin, C.; Pang, Z. Huidouba Improved Podocyte Injury by Down-Regulating Nox4 Expression in Rats with Diabetic Nephropathy. Front. Pharmacol. 2020, 11, 587995. [Google Scholar] [CrossRef]
- Bai, Y.; Shi, D.; Lu, H.; Yang, K.; Zhao, H.; Lu, B.; Pang, Z. Hypoglycemic Effects of Tibetan Medicine Huidouba in STZ-Induced Diabetic Mice and Db/Db Mice. Chin. Herb. Med. 2021, 13, 202–209. [Google Scholar] [CrossRef]
- Gao, H.; Wen, J.-J.; Hu, J.-L.; Nie, Q.-X.; Chen, H.-H.; Xiong, T.; Nie, S.-P.; Xie, M.-Y. Polysaccharide from Fermented Momordica charantia L. with Lactobacillus plantarum NCU116 Ameliorates Type 2 Diabetes in Rats. Carbohydr. Polym. 2018, 201, 624–633. [Google Scholar] [CrossRef]
- Guo, W.-L.; Deng, J.-C.; Pan, Y.-Y.; Xu, J.-X.; Hong, J.-L.; Shi, F.-F.; Liu, G.-L.; Qian, M.; Bai, W.-D.; Zhang, W.; et al. Hypoglycemic and Hypolipidemic Activities of Grifola Frondosa Polysaccharides and Their Relationships with the Modulation of Intestinal Microflora in Diabetic Mice Induced by High-Fat Diet and Streptozotocin. Int. J. Biol. Macromol. 2019, 153, 1231–1240. [Google Scholar] [CrossRef]
- Rangika, B.S.; Dayananda, P.D.; Peiris, D.C. Hypoglycemic and Hypolipidemic Activities of Aqueous Extract of Flowers from Nycantus arbor-tristis L. in Male Mice. BMC Complement. Altern. Med. 2015, 15, 289. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Li, M.; Jiao, Y.; Zeng, M.; He, Z.; Shen, Q.; Chen, J. Effect of Dietary Exposure to Acrylamide on Diabetes-Associated Cognitive Dysfunction from the Perspectives of Oxidative Damage, Neuroinflammation, and Metabolic Disorders. J. Agric. Food Chem. 2022, 70, 4445–4456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative Stress and Diabetes: Antioxidative Strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut Bacteria Selectively Promoted by Dietary Fibers Alleviate Type 2 Diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Salamone, D.; Rivellese, A.A.; Vetrani, C. The Relationship between Gut Microbiota, Short-Chain Fatty Acids and Type 2 Diabetes Mellitus: The Possible Role of Dietary Fibre. Acta Diabetol. 2021, 58, 1131–1138. [Google Scholar] [CrossRef]
- Hagerty, S.L.; Hutchison, K.E.; Lowry, C.A.; Bryan, A.D. An Empirically Derived Method for Measuring Human Gut Microbiome Alpha Diversity: Demonstrated Utility in Predicting Health-Related Outcomes among a Human Clinical Sample. PLoS ONE 2020, 15, e0229204. [Google Scholar] [CrossRef]
- Kelly, B.J.; Gross, R.; Bittinger, K.; Sherrill-Mix, S.; Lewis, J.D.; Collman, R.G.; Bushman, F.D.; Li, H. Power and Sample-Size Estimation for Microbiome Studies Using Pairwise Distances and PERMANOVA. Bioinformatics 2015, 31, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.C.; Collyer, M.L. Permutation Tests for Phylogenetic Comparative Analyses of High-Dimensional Shape Data: What You Shuffle Matters. Evolution 2015, 69, 823–829. [Google Scholar] [CrossRef]
- Huang, F.; Nilholm, C.; Roth, B.; Linninge, C.; Höglund, P.; Nyman, M.; Ohlsson, B. Anthropometric and Metabolic Improvements in Human Type 2 Diabetes after Introduction of an Okinawan-Based Nordic Diet Are Not Associated with Changes in Microbial Diversity or SCFA Concentrations. Int. J. Food Sci. Nutr. 2017, 69, 729–740. [Google Scholar] [CrossRef]
- Murri, M.; Leiva, I.; Gomez-Zumaquero, J.M.; Tinahones, F.J.; Cardona, F.; Soriguer, F.; Queipo-Ortuño, M.I. Gut Microbiota in Children with Type 1 Diabetes Differs from That in Healthy Children: A Case-Control Study. BMC Med. 2013, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Allin, K.H.; Tremaroli, V.; Caesar, R.; Jensen, B.A.H.; Damgaard, M.T.F.; Bahl, M.I.; Licht, T.R.; Hansen, T.H.; Nielsen, T.; Dantoft, T.M.; et al. Aberrant Intestinal Microbiota in Individuals with Prediabetes. Diabetologia 2018, 61, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ni, Y.; Qian, L.; Fang, Q.; Zheng, T.; Zhang, M.; Gao, Q.; Zhang, Y.; Ni, J.; Hou, X.; et al. Decreased Abundance of Akkermansia muciniphila Leads to the Impairment of Insulin Secretion and Glucose Homeostasis in Lean Type 2 Diabetes. Adv. Sci. 2021, 8, 2100536. [Google Scholar] [CrossRef]
- Dong, N.; Yang, X.; Chan, E.W.-C.; Zhang, R.; Chen, S. Klebsiella Species: Taxonomy, Hypervirulence and Multidrug Resistance. eBioMedicine 2022, 79, 103998. [Google Scholar] [CrossRef]
- Zheng, Z.; Lyu, W.; Ren, Y.; Li, X.; Zhao, S.; Yang, H.; Xiao, Y. Allobaculum Involves in the Modulation of Intestinal ANGPTLT4 Expression in Mice Treated by High-Fat Diet. Front. Nutr. 2021, 8, 690138. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-Y.; Zhang, X.-Q.; Chen, A.-L.; Zhang, J.; Lv, B.-H.; Ma, M.-H.; Lian, J.; Wu, Y.-X.; Zhou, Y.-T.; Ma, C.-C.; et al. A Comparative Study of Microbial Community and Functions of Type 2 Diabetes Mellitus Patients with Obesity and Healthy People. Appl. Microbiol. Biotechnol. 2020, 104, 7143–7153. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Yang, L.; Huang, P.; Li, W.; Wang, S.; Zhao, G.; Zhang, M.; Pang, X.; Yan, Z.; et al. Structural Modulation of Gut Microbiota in Life-Long Calorie-Restricted Mice. Nat. Commun. 2013, 4, 2163. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Lv, Y.; Jiang, Y.; Pan, J.; Zhu, Y.; Huang, M.; Zhang, S. Changes in Intestinal Microbiota of Type 2 Diabetes in Mice in Response to Dietary Supplementation with Instant Tea or Matcha. Can. J. Diabetes 2019, 44, 44–52. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, L.; Zhou, H.; Deng, C.; Han, Q.; Chen, Y.; Wu, Q.; Li, S. Protective Effect of Carvacrol on Liver Injury in Type 2 Diabetic Db/Db Mice. Mol. Med. Rep. 2021, 24, 741. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ding, Q.; Nie, S.-P.; Zhang, Y.-S.; Xiong, T.; Xie, M.-Y. Carrot Juice Fermented with Lactobacillus plantarum NCU116 Ameliorates Type 2 Diabetes in Rats. J. Agric. Food Chem. 2014, 62, 11884–11891. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the Gut Microbiome and Gastrointestinal Health in Humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Aronson, D.; Edelman, E.R. Coronary Artery Disease and Diabetes Mellitus. Cardiol. Clin. 2014, 32, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Yissachar, N.; Zhou, Y.; Ung, L.; Lai, N.Y.; Mohan, J.F.; Ehrlicher, A.; Weitz, D.A.; Kasper, D.L.; Chiu, I.M.; Mathis, D.; et al. An Intestinal Organ Culture System Uncovers a Role for the Nervous System in Microbe-Immune Crosstalk. Cell 2017, 168, 1135–1148.e12. [Google Scholar] [CrossRef]
- Ramezani, A.; Massy, Z.A.; Meijers, B.; Evenepoel, P.; Vanholder, R.; Raj, D.S. Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target. Am. J. Kidney Dis. 2015, 67, 483–498. [Google Scholar] [CrossRef]
- Das, T.; Jayasudha, R.; Chakravarthy, S.; Prashanthi, G.S.; Bhargava, A.; Tyagi, M.; Rani, P.K.; Pappuru, R.R.; Sharma, S.; Shivaji, S. Alterations in the Gut Bacterial Microbiome in People with Type 2 Diabetes Mellitus and Diabetic Retinopathy. Sci. Rep. 2021, 11, 2738. [Google Scholar] [CrossRef]
- Cani, P.D.; Osto, M.; Geurts, L.; Everard, A. Involvement of Gut Microbiota in the Development of Low-Grade Inflammation and Type 2 Diabetes Associated with Obesity. Gut Microbes 2012, 3, 279–288. [Google Scholar] [CrossRef]
- Nie, Q.; Hu, J.; Chen, H.; Geng, F.; Nie, S. Arabinoxylan Ameliorates Type 2 Diabetes by Regulating the Gut Microbiota and Metabolites. Food Chem. 2021, 371, 131106. [Google Scholar] [CrossRef]
- Berger, K.; Burleigh, S.; Lindahl, M.; Bhattacharya, A.; Patil, P.; Stålbrand, H.; Nordberg Karlsson, E.; Hållenius, F.; Nyman, M.; Adlercreutz, P. Xylooligosaccharides Increase Bifidobacteria and Lachnospiraceae in Mice on a High-Fat Diet, with a Concomitant Increase in Short-Chain Fatty Acids, Especially Butyric Acid. J. Agric. Food Chem. 2021, 69, 3617–3625. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, S.; Tang, L.; Zhang, M.; Yuan, M.; Wang, P.; Gao, X. Integrative Analysis of Fecal Metabolome and Gut Microbiota in High-Fat Diet-Induced Hyperlipidemic Rats Treated with Rosa Roxburghii Tratt Juice. J. Funct. Foods 2022, 90, 104978. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, M.; Feng, D.; Zhang, Y.; Zuo, Y.; Li, J.; Wang, L.; Hu, P. Effect and Correlation of Rosa roxburghii Tratt Juice Fermented by Lactobacillus paracasei SR10-1 on Oxidative Stress and Gut Microflora Dysbiosis in Streptozotocin (STZ)-Induced Type 2 Diabetes Mellitus Mice. Foods 2023, 12, 3233. https://doi.org/10.3390/foods12173233
Wei M, Feng D, Zhang Y, Zuo Y, Li J, Wang L, Hu P. Effect and Correlation of Rosa roxburghii Tratt Juice Fermented by Lactobacillus paracasei SR10-1 on Oxidative Stress and Gut Microflora Dysbiosis in Streptozotocin (STZ)-Induced Type 2 Diabetes Mellitus Mice. Foods. 2023; 12(17):3233. https://doi.org/10.3390/foods12173233
Chicago/Turabian StyleWei, Maoyang, Dandan Feng, Yulong Zhang, Yunyang Zuo, Jiuchang Li, Ling Wang, and Ping Hu. 2023. "Effect and Correlation of Rosa roxburghii Tratt Juice Fermented by Lactobacillus paracasei SR10-1 on Oxidative Stress and Gut Microflora Dysbiosis in Streptozotocin (STZ)-Induced Type 2 Diabetes Mellitus Mice" Foods 12, no. 17: 3233. https://doi.org/10.3390/foods12173233