Pulsed Electric Field-Assisted Enzymatic and Alcoholic–Alkaline Production of Porous Granular Cold-Water-Soluble Starch: A Carrier with Efficient Zeaxanthin-Loading Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
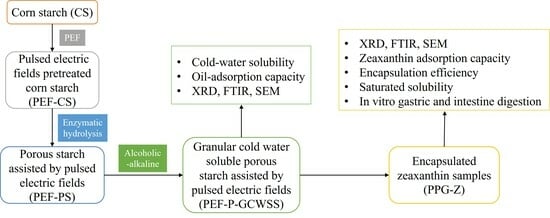
2.2. Preparation of Different Starch Samples
- (1)
- Pulsed electric field-treated corn starch (PEF-CS): Corn starch (CS) was dissolved in deionized water with a concentration of 15% (m/m). Potassium chloride solution (0.5 mol/L) was added to the starch dispersion to increase the conductivity of the starch dispersion to 150 ± 5 μS/cm. The starch dispersion was pumped into the PEF treatment equipment [11] at a constant flow rate of 2 mL/s. The corn starch was pretreated and modified under the electric field intensity of 12 kV/cm and an effective treatment time of 18 ms. The starch emulsion was centrifuged to obtain a precipitated sample, washed 3 times with deionized water, vacuum filtered, dried, and ground into powder to obtain pulsed electric fields-pretreated corn starch (PEF-CS).
- (2)
- Porous starch (PS): Corn starch was dissolved in acetic acid sodium acetate (Hac-NaAc, pH = 5.0) buffer solution with a concentration of 30% (m/m). Starch dispersion was stirred and activated for 30 min under water bath conditions at 50 °C. The enzymatic hydrolysis method was slightly modified based on the method proposed by Chen et al. [26]. α-amylase and glucose amylase were prepared as composite enzymes in a ratio of 1:1. The sample was enzymatically hydrolyzed for 4.5 h with an enzyme concentration of 2.0%. The precipitates were obtained by centrifuging the sample at 8000 rpm/min for 20 min and were washed 4 times with deionized water and anhydrous ethanol, respectively. After vacuum filtration, drying, and grinding of the precipitated sample, porous starch samples (PS) were obtained.
- (3)
- Pulsed electric field-treated porous starch (PEF-PS): Similar to the preparation method of PS, porous starch assisted by pulsed electric fields (PEF-PS) was obtained by replacing corn starch with PEF-CS.
- (4)
- Granular cold-water-soluble starch (GCWSS): Corn starch (CS) was modified with the alcoholic–alkaline method [27,28]. Corn starch was mixed with 80% ethanol to form a starch dispersion and stirred evenly in a 25 °C water bath. An amount of 4.8 wt% sodium hydroxide was added to the system, and the pH was adjusted to neutral after a reaction time of 15 min. The sample was washed four times with anhydrous ethanol, vacuum filtered, and dried to a constant weight to obtain granular cold-water-soluble starch (GCWSS).
- (5)
- Pulsed electric field-treated granular cold-water-soluble starch (PEF-GCWSS): Similar to the preparation method of GCWSS, pulsed electric fields-pretreated granule cold-water-soluble starch (PEF-GCWSS) was obtained by replacing corn starch with PEF-CS.
- (6)
- Porous granular cold-water-soluble starch (P-GCWSS): Similar to the preparation method of GCWSS, granule cold-water-soluble porous starch (P-GCWSS) [15] was obtained by replacing corn starch with PS.
- (7)
- Pulsed electric field-treated porous granular cold-water-soluble starch (PEF-P-GCWSS): Similar to the preparation method of GCWSS, granular cold-water-soluble porous starch assisted by pulsed electric fields (PEF-P-GCWSS) was obtained by replacing corn starch with PEF-PS.
2.3. Preparation of Starch–Zeaxanthin Composites
2.4. Cold Water Solubility and Oil-Adsorption Capacity
2.5. Scanning Electron Microscopy (SEM)
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.7. X-ray Diffraction (XRD)
2.8. Zeaxanthin Adsorption Capacity and Encapsulation Efficiency
2.9. Determination of Saturated Solubility
2.10. Storage Stability
2.11. In Vitro Gastric and Intestine Digestion
2.12. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of the Starch Samples
3.1.1. Cold Water Solubility and Oil Adsorption Rate Analysis
3.1.2. SEM Analysis
3.1.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.1.4. X-ray Diffraction (XRD) Analysis
3.2. Physicochemical Characterization of the Starch–Zeaxanthin Samples
3.2.1. Analysis of the Zeaxanthin Adsorption Capacity and Encapsulation Efficiency
3.2.2. SEM Analysis
3.2.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.2.4. X-ray Diffraction (XRD) Analysis
3.3. The Saturated Solubility Analysis
3.4. Storage Stability Analysis
3.5. In Vitro Gastric and Intestine Digestion
4. Conclusions
- (1)
- The similarities and differences in the preparation of PEF-P-GCWSS from starch granules with different branched chains, different crystalline forms, and different plant sources;
- (2)
- Structural and physicochemical property changes of PEF-PS under other methods of preparing granular cold-water-soluble starch;
- (3)
- Adsorptive encapsulation of different structural guests by PEF-P-GCWSS.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, F.; Lu, S.; Wang, L.; Zheng, M.; Young Quek, S. Modified porous starch for enhanced properties: Synthesis, characterization and applications. Food Chem. 2023, 415, 135765. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Y.; Liu, J.; Xu, X. Preparation, characterization, physicochemical property and potential application of porous starch: A review. Int. J. Biol. Macromol. 2020, 148, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, M.-N.; Chen, H.-Q.; Zhang, B. Effects of the combination of repeated heat-moisture treatment and compound enzymes hydrolysis on the structural and physicochemical properties of porous wheat starch. Food Chem. 2019, 274, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Benavent-Gil, Y.; Rosell, C.M. Morphological and physicochemical characterization of porous starches obtained from different botanical sources and amylolytic enzymes. Int. J. Biol. Macromol. 2017, 103, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cui, D.; Liu, M.; Gong, H.; Huang, Y.; Han, F. Corn porous starch: Preparation, characterization and adsorption property. Int. J. Biol. Macromol. 2012, 50, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Piloni, R.V.; Bordón, M.G.; Barrera, G.N.; Martínez, M.L.; Ribotta, P.D. Porous Microparticles of Corn Starch as Bio-Carriers for Chia Oil. Foods 2022, 11, 4022. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lv, J.; Jiang, S.; Niu, B.; Pang, M.; Jiang, S. Preparation and characterization of porous corn starch and its adsorption toward grape seed proanthocyanidins. Starch-Stärke 2016, 68, 1254–1263. [Google Scholar] [CrossRef]
- Benavent-Gil, Y.; Rodrigo, D.; Rosell, C.M. Thermal stabilization of probiotics by adsorption onto porous starches. Carbohydr. Polym. 2018, 197, 558–564. [Google Scholar] [CrossRef]
- Han, Z.; Zeng, X.-A.; Zhang, B.-S.; Yu, S.-J. Effects of pulsed electric fields (PEF) treatment on the properties of corn starch. J. Food Eng. 2009, 93, 318–323. [Google Scholar] [CrossRef]
- Han, Z.; Zeng, X.A.; Fu, N.; Yu, S.J.; Chen, X.D.; Kennedy, J.F. Effects of pulsed electric field treatments on some properties of tapioca starch. Carbohydr. Polym. 2012, 89, 1012–1017. [Google Scholar] [CrossRef]
- Han, Z.; Han, Y.; Wang, J.; Liu, Z.; Buckow, R.; Cheng, J. Effects of pulsed electric field treatment on the preparation and physicochemical properties of porous corn starch derived from enzymolysis. J. Food Process. Preserv. 2020, 44, e14353. [Google Scholar] [CrossRef]
- Chen, B.-R.; Wen, Q.-H.; Zeng, X.-A.; Abdul, R.; Roobab, U.; Xu, F.-Y. Pulsed electric field assisted modification of octenyl succinylated potato starch and its influence on pasting properties. Carbohydr. Polym. 2021, 254, 117294. [Google Scholar] [CrossRef] [PubMed]
- Majzoobi, M.; Kaveh, Z.; Blanchard, C.L.; Farahnaky, A. Physical properties of pregelatinized and granular cold water swelling maize starches in presence of acetic acid. Food Hydrocoll. 2015, 51, 375–382. [Google Scholar] [CrossRef]
- BeMiller, J.N.; Huber, K.C. Physical Modification of Food Starch Functionalities. Annu. Rev. Food Sci. Technol. 2015, 6, 19–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dai, G.; Gao, Q. Preparation and properties of granular cold-water-soluble porous starch. Int. J. Biol. Macromol. 2020, 144, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Champrasert, O.; Sagis, L.M.C.; Suwannaporn, P. Emulsion-based oleogelation using octenyl succinic anhydride modified granular cold-water swelling starch. Food Hydrocoll. 2023, 135, 108186. [Google Scholar] [CrossRef]
- Zhou, X.; Chang, Q.; Li, J.; Jiang, L.; Xing, Y.; Jin, Z. Preparation of V-type porous starch by amylase hydrolysis of V-type granular starch in aqueous ethanol solution. Int. J. Biol. Macromol. 2021, 183, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-A.; Lim, S.-T. Structural changes in corn starches during alkaline dissolution by vortexing. Carbohydr. Polym. 2004, 55, 193–199. [Google Scholar] [CrossRef]
- Shi, L.; Fu, X.; Tan, C.P.; Huang, Q.; Zhang, B. Encapsulation of Ethylene Gas into Granular Cold-Water-Soluble Starch: Structure and Release Kinetics. J. Agric. Food Chem. 2017, 65, 2189–2197. [Google Scholar] [CrossRef]
- Gao, Q.; Sun, Y.; He, R.; Zheng, J.; Zhang, B.; Tan, C.P.; Fu, X.; Huang, Q. Molecular encapsulation of cinnamaldehyde in V-type starch: The role of solvent and temperature. Food Hydrocoll. 2023, 136, 108285. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; López-Pozo, M.; Stewart, J.J.; Adams, W.W. Zeaxanthin and Lutein: Photoprotectors, Anti-Inflammatories, and Brain Food. Molecules 2020, 25, 3607. [Google Scholar] [CrossRef]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Feng, D.; Li, E.; Gilbert, R.G. Formation, Structural Characterization, and Functional Properties of Corn Starch/Zeaxanthin Composites. Foods 2023, 12, 2076. [Google Scholar] [CrossRef] [PubMed]
- de Campo, C.; Dick, M.; dos Santos, P.P.; Haas Costa, T.M.; Paese, K.; Stanisçuaski Guterres, S.; de Oliveira Rios, A.; Hickmann Flôres, S. Zeaxanthin nanoencapsulation with Opuntia monacantha mucilage as structuring material: Characterization and stability evaluation under different temperatures. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 410–421. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, S.; Tang, Z.; Chen, X.; Zhang, Z. Structural changes of cassava starch granules hydrolyzed by a mixture of α-amylase and glucoamylase. Carbohydr. Polym. 2011, 85, 272–275. [Google Scholar] [CrossRef]
- Butt, N.A.; Ali, T.M.; Hasnain, A. Rheological characterization of cold water soluble rice (Oryza sativa) starch lactates and citrates prepared via alcoholic-alkaline method. Int. J. Biol. Macromol. 2019, 123, 558–568. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Baik, M.-Y.; Kim, B.-Y. Characteristics of granular cold-water-soluble potato starch treated with alcohol and alkali. Food Sci. Biotechnol. 2017, 26, 1263–1270. [Google Scholar] [CrossRef]
- Shi, L.; Li, Z.; Guo, J.; Kong, L.; Ren, Z.; Weng, W. Encapsulation and release kinetics of ethylene into “pre-formed” V-type starch and granular cold-water-soluble starch. Carbohydr. Polym. 2022, 287, 119360. [Google Scholar] [CrossRef]
- Cheng, W.; Luo, Z.; Li, L.; Fu, X. Preparation and Characterization of Debranched-Starch/Phosphatidylcholine Inclusion Complexes. J. Agric. Food Chem. 2015, 63, 634–641. [Google Scholar] [CrossRef]
- Fang, C.; Huang, J.; Pu, H.; Yang, Q.; Chen, Z.; Zhu, Z. Cold-water solubility, oil-adsorption and enzymolysis properties of amorphous granular starches. Food Hydrocoll. 2021, 117, 106669. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Wang, Y.; Lin, D.; Li, X.; Liu, J. Preparation and Properties of Granular Cold-Water-Soluble Maize Starch by Ultrasonic-Assisted Alcoholic-Alkaline Treatment. Starch-Stärke 2018, 70, 1700354. [Google Scholar] [CrossRef]
- Lin, J.; Tang, Z.-S.; Brennan, C.S.; Zeng, X.-A. Thermomechanically micronized sugar beet pulp: Dissociation mechanism, physicochemical characteristics, and emulsifying properties. Food Res. Int. 2022, 160, 111675. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sun, W.; Meng, Z.; Zhu, X.; Gan, H.; Gu, R.; Wu, Z.; Dou, G. Preparation and characterization of a new type of porous starch microspheres (PSM) and effect of physicochemical properties on water uptake rate. Int. J. Biol. Macromol. 2018, 116, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, C.; Fu, X.; Li, C.; He, X.; Zhang, B.; Huang, Q. Encapsulation of lutein into swelled cornstarch granules: Structure, stability and in vitro digestion. Food Chem. 2018, 268, 362–368. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Designing Food Structure to Control Stability, Digestion, Release and Absorption of Lipophilic Food Components. Food Biophys. 2008, 3, 219–228. [Google Scholar] [CrossRef]
- Marefati, A.; Bertrand, M.; Sjöö, M.; Dejmek, P.; Rayner, M. Storage and digestion stability of encapsulated curcumin in emulsions based on starch granule Pickering stabilization. Food Hydrocoll. 2017, 63, 309–320. [Google Scholar] [CrossRef]
- Jivan, M.J.; Yarmand, M.; Madadlou, A. Preparation of cold water-soluble potato starch and its characterization. J. Food Sci. Technol. 2014, 51, 601–605. [Google Scholar] [CrossRef]
- Fang, C.; Huang, J.; Yang, Q.; Pu, H.; Liu, S.; Zhu, Z. Adsorption capacity and cold-water solubility of honeycomb-like potato starch granule. Int. J. Biol. Macromol. 2020, 147, 741–749. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, J.; Gao, W. Process optimization of ultrasound-assisted alcoholic-alkaline treatment for granular cold water swelling starches. Ultrason. Sonochem. 2017, 38, 579–584. [Google Scholar] [CrossRef]
- Bello-Pérez, L.A.; Romero-Manilla, R.; Paredes-López, O. Preparation and Properties of Physically Modified Banana Starch Prepared by Alcoholic-Alkaline Treatment. Starch-Stärke 2000, 52, 154–159. [Google Scholar] [CrossRef]
- Kong, L.; Ziegler, G.R. Molecular encapsulation of ascorbyl palmitate in preformed V-type starch and amylose. Carbohydr. Polym. 2014, 111, 256–263. [Google Scholar] [CrossRef]
- Nalawade, P.; Gajjar, A. Preparation and characterization of spray dried complexes of lutein with cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2015, 83, 77–87. [Google Scholar] [CrossRef]
- Lin, J.; Tang, Z.-S.; Brennan, C.S.; Chandrapala, J.; Gao, W.; Han, Z.; Zeng, X.-A. Valorizing protein-polysaccharide conjugates from sugar beet pulp as an emulsifier. Int. J. Biol. Macromol. 2023, 226, 679–689. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, G.; Wanbin, Z.; Minghao, J.; Wei, Y.; Hao, J.; Liu, X.; Gan, Z.; Sun, A. Nanoencapsulation of zeaxanthin extracted from Lycium barbarum L. by complex coacervation with gelatin and CMC. Food Hydrocoll. 2021, 112, 106280. [Google Scholar] [CrossRef]
- Lin, J.; Tang, Z.-S.; Chandrapala, J.; Brennan, C.S.; Han, Z.; Zeng, X.-A. Elucidation of the cellulose nanocrystal-sugar beet pectin interactions for emulsification enhancement. Food Hydrocoll. 2023, 135, 108198. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, H.; Liao, Z.; Wang, L.; Zeng, X.; Han, Z. Pulsed Electric Field-Assisted Enzymatic and Alcoholic–Alkaline Production of Porous Granular Cold-Water-Soluble Starch: A Carrier with Efficient Zeaxanthin-Loading Capacity. Foods 2023, 12, 3189. https://doi.org/10.3390/foods12173189
Lei H, Liao Z, Wang L, Zeng X, Han Z. Pulsed Electric Field-Assisted Enzymatic and Alcoholic–Alkaline Production of Porous Granular Cold-Water-Soluble Starch: A Carrier with Efficient Zeaxanthin-Loading Capacity. Foods. 2023; 12(17):3189. https://doi.org/10.3390/foods12173189
Chicago/Turabian StyleLei, Huanqing, Zhongjuan Liao, Langhong Wang, Xinan Zeng, and Zhong Han. 2023. "Pulsed Electric Field-Assisted Enzymatic and Alcoholic–Alkaline Production of Porous Granular Cold-Water-Soluble Starch: A Carrier with Efficient Zeaxanthin-Loading Capacity" Foods 12, no. 17: 3189. https://doi.org/10.3390/foods12173189