SERS with Flexible β-CD@AuNP/PTFE Substrates for In Situ Detection and Identification of PAH Residues on Fruit and Vegetable Surfaces Combined with Lightweight Network
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
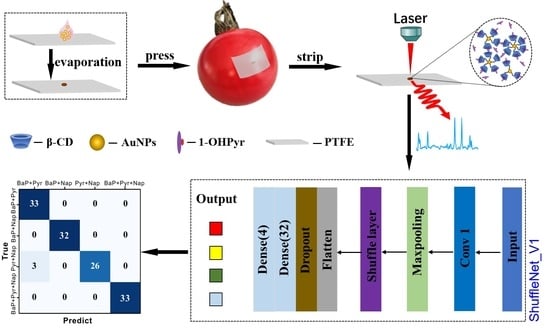
2.2. Preparation of Flexible SERS Substrate
2.3. Preparation of SERS Sample
2.4. Spectral Measurement
2.5. Spectral Analysis Methods
2.6. Model Evaluation
3. Results and Discussion
3.1. Influence of Different Flexible Substrates on SERS Activity
3.2. SERS Detection of PAHs Based on Flexible β-CD@AuNP/PTFE
3.3. In Situ Detection of PAHs on Fruit and Vegetable Surfaces
3.4. Identification of Various PAHs on Fruit and Vegetable Surfaces
3.5. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lawal, A.T. Polycyclic aromatic hydrocarbons. A review. Cogent Environ. Sci. 2017, 3, 1339841. [Google Scholar] [CrossRef]
- Han, J.; Liang, Y.; Zhao, B.; Wang, Y.; Xing, F.; Qin, L. Polycyclic aromatic hydrocarbon (PAHs) geographical distribution in China and their source, risk assessment analysis. Environ. Pollut. 2019, 251, 312–327. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci. Total Environ. 2019, 696, 133971. [Google Scholar] [CrossRef]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A. Polycyclic aromatic hydrocarbons in foods: Biological effects, legislation, occurrence, analytical methods, and strategies to reduce their formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, Y.; Hou, J.; Zheng, D.; Wang, G.; Hu, C.; Xu, T.; Cheng, J.; Yin, W.; Mao, X.; et al. Associations between inhaled doses of PM2.5-bound polycyclic aromatic hydrocarbons and fractional exhaled nitric oxide. Chemosphere 2019, 218, 992–1001. [Google Scholar] [CrossRef]
- Li, G.; Wu, S.; Wang, L.; Akoh, C.C. Concentration, dietary exposure and health risk estimation of polycyclic aromatic hydrocarbons (PAHs) in youtiao, a Chinese traditional fried food. Food Control 2016, 59, 328–336. [Google Scholar] [CrossRef]
- Ashayeri, N.Y.; Keshavarzi, B.; Moore, F.; Kersten, M.; Yazdi, M.; Lahijanzadeh, A.R. Presence of polycyclic aromatic hydrocarbons in sediments and surface water from Shadegan wetland–Iran: A focus on source apportionment, human and ecological risk assessment and sediment-water exchange. Ecotox. Environ. Saf. 2018, 148, 1054–1066. [Google Scholar] [CrossRef]
- Sun, N.; Yu, S.; Cai, Z.; Liu, J.; Wang, T.; Qi, B.; Wang, Z.; Wang, S.; Yang, A.; Zhu, G.; et al. Inhibition of polycyclic aromatic hydrocarbon (PAHs) release from sediments in an integrated rice and crab coculture system by rice straw biochar. J. Clean. Prod. 2022, 367, 133058. [Google Scholar] [CrossRef]
- Ma, C.M.; Lin, L.C.; Chuang, K.J.; Hong, G.B. Colorimetric detection of polycyclic aromatic hydrocarbons by using gold nanoparticles. Spectrochim. Acta A 2022, 268, 120701. [Google Scholar] [CrossRef]
- Pena, E.A.; Ridley, L.M.; Murphy, W.R.; Sowa, J.R.; Bentivegna, C.S. Detection of polycyclic aromatic hydrocarbons (PAHs) in raw menhaden fish oil using fluorescence spectroscopy: Method development. Environ. Toxicol. Chem. 2015, 34, 1946–1958. [Google Scholar] [CrossRef]
- Dong, G.; Li, X.; Yang, R.; Yang, Y.; Liu, H.; Wu, N. Correction method of effect of soil moisture on the fluorescence intensity of polycyclic aromatic hydrocarbons based on near-infrared diffuse reflection spectroscopy. Environ. Pollut. 2021, 269, 116150. [Google Scholar] [CrossRef]
- Eremina, O.E.; Sergeeva, E.A.; Ferree, M.V.; Shekhovtsova, T.N.; Goodilin, E.A.; Veselova, I.A. Dual-Purpose SERS Sensor for Selective Determination of Polycyclic Aromatic Compounds via Electron Donor-Acceptor Traps. ACS Sens. 2021, 6, 1057–1066. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A review on surface-enhanced Raman scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Schlücker, S. Surface-Enhanced raman spectroscopy: Concepts and chemical applications. Angew. Chem. Int. Edit. 2014, 53, 4756–4795. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lorente, A.I. Recent developments on gold nanostructures for surface enhanced Raman spectroscopy: Particle shape, substrates and analytical applications. A review. Anal. Chim. Acta 2021, 1168, 338474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Pu, H.; Huang, L.; Sun, D.W. Advances in flexible surface-enhanced Raman scattering (SERS) substrates for nondestructive food detection: Fundamentals and recent applications. Trends Food Sci. Tec. 2021, 109, 690–701. [Google Scholar] [CrossRef]
- Gao, Y.; Li, L.; Zhang, X.; Wang, X.; Ji, W.; Zhao, J.; Ozaki, Y. CTAB-triggered Ag aggregates for reproducible SERS analysis of urinary polycyclic aromatic hydrocarbon metabolites. Chem. Commun. 2019, 55, 2146–2149. [Google Scholar] [CrossRef] [PubMed]
- Poonia, M.; Kuster, T.; Bothun, G.D. Organic Anion Detection with Functionalized SERS Substrates via Coupled Electrokinetic Preconcentration, Analyte Capture, and Charge Transfer. ACS Appl. Mater. Interfaces 2022, 14, 23964–23972. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Zheng, S.; Li, P.; Tang, L.; Xu, Q.; Weng, S. Detection of 1-OHPyr in human urine using SERS with injection under wet liquid–liquid self-assembled films of β-CD-coated gold nanoparticles and deep learning. Spectrochim. Acta A 2022, 290, 122238. [Google Scholar] [CrossRef]
- Alyami, A.; Quinn, A.J.; Iacopino, D. Flexible and transparent Surface Enhanced Raman Scattering (SERS)-Active Ag NPs/PDMS composites for in-situ detection of food contaminants. Talanta 2019, 201, 58–64. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Kannan, P.; Zhang, L.; Lin, Z.; Zhang, J.; Chen, T.; Guo, L. Flexible and adhesive surface enhance Raman scattering active tape for rapid detection of pesticide residues in fruits and vegetables. Anal. Chem. 2016, 88, 2149–2155. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.B.; Czaplicka, M.; Kowalska, A.A.; Szymborski, T.; Kamińska, A. Flexible PET/ITO/Ag SERS Platform for Label-Free Detection of Pesticides. Biosensors 2019, 9, 111. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, M.; Liu, Z.; Bian, W.; Zhang, X.; Zhan, J. Hydrophobic silver nanowire membrane for swabbing extraction and in situ SERS detection of polycyclic aromatic hydrocarbons on toys. Anal. Methods 2017, 9, 1816–1824. [Google Scholar] [CrossRef]
- Zhang, D.; You, H.; Yuan, L.; Hao, R.; Li, T.; Fang, J. Hydrophobic slippery surface-based surface-enhanced Raman spectroscopy platform for ultrasensitive detection in food safety applications. Anal. Chem. 2019, 91, 4687–4695. [Google Scholar] [CrossRef] [PubMed]
- Kasani, S.; Curtin, K.; Wu, N. A review of 2D and 3D plasmonic nanostructure array patterns: Fabrication, light management and sensing applications. Nanophotonics 2019, 8, 2065–2089. [Google Scholar] [CrossRef]
- Huang, C.Y.; Tsai, M.S. Fabrication of 3D nano-hemispherical cavity array plasmonic substrate for SERS applications. Int. J. Optomechatron. 2018, 12, 40–52. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Q.; Yin, M.; Yang, D.; Yang, Y. A super-hydrophobic perfluoropolyether coated polytetrafluoroethylene sheets substrate for detection of acetamiprid surface-enhanced Raman spectroscopy. Spectrochim. Acta A 2022, 278, 121373. [Google Scholar] [CrossRef]
- Ciloglu, F.U.; Caliskan, A.; Saridag, A.M.; Kilic, I.H.; Tokmakci, M.; Kahraman, M.; Aydin, O. Drug-resistant Staphylococcus aureus bacteria detection by combining surface-enhanced Raman spectroscopy (SERS) and deep learning techniques. Sci. Rep. 2021, 11, 18444. [Google Scholar] [CrossRef]
- Weng, S.; Zhu, W.; Li, P.; Yuan, H.; Zhang, X.; Zheng, L.; Zhao, J.; Huang, L.; Han, P. Dynamic surface-enhanced Raman spectroscopy for the detection of acephate residue in rice by using gold nanorods modified with cysteamine and multivariant methods. Food Chem. 2020, 310, 125855. [Google Scholar] [CrossRef]
- Gomes, R.; Rozario, P.; Adhikari, N. Deep Learning Optimization in Remote Sensing Image Segmentation using Dilated Convolutions and ShuffleNet. In Proceedings of the 2021 IEEE International Conference on Electro Information Technology (EIT), Mt. Pleasant, MI, USA, 14–15 May 2021; pp. 244–249. [Google Scholar]
- Zhou, Y.; Chen, S.; Wang, Y.; Huan, W. Review of Research on Lightweight Convolutional Neural Networks. In Proceedings of the 2020 IEEE 5th Information Technology and Mechatronics Engineering Conference, Chongqing, China, 12–14 June 2020; pp. 1713–1720. [Google Scholar] [CrossRef]
- Asghar, M.Z.; Albogamy, F.R.; Al-Rakhami, M.S.; Asghar, J.; Rahmat, M.K.; Alam, M.M.; Lajis, A.; Nasir, H.M. Facial mask detection using depthwise separable convolutional neural network model during COVID-19 pandemic. Front. Public Health 2022, 10, 855254. [Google Scholar] [CrossRef]
- Lussier, F.; Thibault, V.; Charron, B.; Wallace, G.Q.; Masson, J.F. Deep learning and artificial intelligence methods for Raman and surface-enhanced Raman scattering. TrAC 2020, 124, 115796. [Google Scholar] [CrossRef]
- Weng, S.; Tang, L.; Qiu, M.; Wang, J.; Wu, Y.; Zhu, R.; Wang, C.; Li, P.; Sha, W.; Liang, D. Surface-enhanced Raman spectroscopy charged probes under inverted superhydrophobic platform for detection of agricultural chemicals residues in rice combined with lightweight deep learning network. Anal. Chim. Acta 2023, 1262, 341264. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, F.; Wang, R.; Guo, J.; Zhang, C.; Liu, H.; Wang, Y.; Zong, G.; Feng, W. A Lightweight convolutional neural network for nicotine prediction in tobacco by near-infrared spectroscopy. Front. Plant Sci. 2023, 14, 1138693. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, Y.; Zhu, H.; Zhu, Q.; Xia, Y. Three-in-one: Sensing, self-assembly, and cascade catalysis of cyclodextrin modified gold nanoparticles. J. Am. Chem. Soc. 2016, 138, 16645–16654. [Google Scholar] [CrossRef]
- Karthik, T.S.; Krishna, R.V.V.; Ramakrishna Rao, T.K.; Manoranjithem, V.; Kalaiarasi, S.; Jegajothi, B. Evolutionary Optimization Algorithm on Content Based Image Retrieval System using Handcrafted Features with Squeeze Networks. In Proceedings of the 2022 Second International Conference on Artificial Intelligence and Smart Energy, Coimbatore, India, 23–25 February 2022; pp. 1425–1431. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Zou, T.; Wang, X.; You, J.; Luo, Y. A novel image classification approach via dense-MobileNet models. Mob. Inf. Syst. 2020, 2020, 7602384. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, Z.; Zhu, K. An Optimized CNN Model for Engagement Recognition in an E-Learning Environment. Appl. Sci. 2022, 12, 8007. [Google Scholar] [CrossRef]
- Tao, M.; Fang, H.; Feng, X.; He, Y.; Liu, X.; Shi, Y.; Wei, Y.; Hong, Z. Rapid Trace Detection of Pesticide Residues on Tomato by Surface-Enhanced Raman Spectroscopy and Flexible Tapes. J. Food Qual. 2022, 2022, 6947775. [Google Scholar] [CrossRef]
- Beeram, R.; Vendamani, V.S.; Soma, V.R. Deep learning approach to overcome signal fluctuations in SERS for efficient On-Site trace explosives detection. Spectrochim. Acta A 2023, 289, 122218. [Google Scholar] [CrossRef]
- Erzina, M.; Trelin, A.; Guselnikova, O.; Dvorankova, B.; Strnadova, K.; Perminova, A.; Ulbrich, P.; Mares, D.; Jerabek, V.; Elashnikov, R.; et al. Precise cancer detection via the combination of functionalized SERS surfaces and convolutional neural network with independent inputs. Sensor. Actuat. B-Chem. 2020, 308, 127660. [Google Scholar] [CrossRef]
- Yu, H.; Yang, Z.; Fu, S.; Zhang, Y.; Panneerselvamc, R.; Li, B.; Zhang, L.; Chen, Z.; Wang, X.; Li, J. Intelligent convolution neural network-assisted SERS to realize highly accurate identification of six pathogenic Vibrio. Chem. Commun. 2023, 59, 5779–5782. [Google Scholar] [CrossRef]






| Methods | Classes | Accuracy (%) | Prediction Dataset | ||
|---|---|---|---|---|---|
| Precision (%) | Recall (%) | F1-Score (%) | |||
| Squeezenet | BaP + Pyr | ACCT = 99.57 ACCV = 93.22 ACCP = 94.48 | 96.97 | 84.21 | 90.14 |
| BaP + Nap | 93.75 | 96.77 | 95.24 | ||
| Pyr + Nap | 86.21 | 100 | 92.59 | ||
| BaP + Pyr +Nap | 100 | 100 | 100 | ||
| Mobilenet_V1 | BaP + Pyr | ACCT = 100 ACCV = 94.92 ACCP = 96.06 | 100 | 93.94 | 96.88 |
| BaP + Nap | 96.88 | 100 | 98.42 | ||
| Pyr + Nap | 86.21 | 100 | 92.59 | ||
| BaP+Pyr+Nap | 100 | 86.84 | 92.96 | ||
| Shufflenet_V1 | BaP + Pyr | ACCT = 100 ACCV = 96.61 ACCP = 97.63 | 100 | 91.67 | 95.65 |
| BaP + Nap | 100 | 100 | 100 | ||
| Pyr + Nap | 89.66 | 100 | 94.55 | ||
| BaP + Pyr + Nap | 100 | 100 | 100 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, M.; Tang, L.; Wang, J.; Xu, Q.; Zheng, S.; Weng, S. SERS with Flexible β-CD@AuNP/PTFE Substrates for In Situ Detection and Identification of PAH Residues on Fruit and Vegetable Surfaces Combined with Lightweight Network. Foods 2023, 12, 3096. https://doi.org/10.3390/foods12163096
Qiu M, Tang L, Wang J, Xu Q, Zheng S, Weng S. SERS with Flexible β-CD@AuNP/PTFE Substrates for In Situ Detection and Identification of PAH Residues on Fruit and Vegetable Surfaces Combined with Lightweight Network. Foods. 2023; 12(16):3096. https://doi.org/10.3390/foods12163096
Chicago/Turabian StyleQiu, Mengqing, Le Tang, Jinghong Wang, Qingshan Xu, Shouguo Zheng, and Shizhuang Weng. 2023. "SERS with Flexible β-CD@AuNP/PTFE Substrates for In Situ Detection and Identification of PAH Residues on Fruit and Vegetable Surfaces Combined with Lightweight Network" Foods 12, no. 16: 3096. https://doi.org/10.3390/foods12163096