Microencapsulation of Betaxanthin Pigments from Pitahaya (Hylocereus megalanthus) By-Products: Characterization, Food Application, Stability, and In Vitro Gastrointestinal Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Vegetal Material
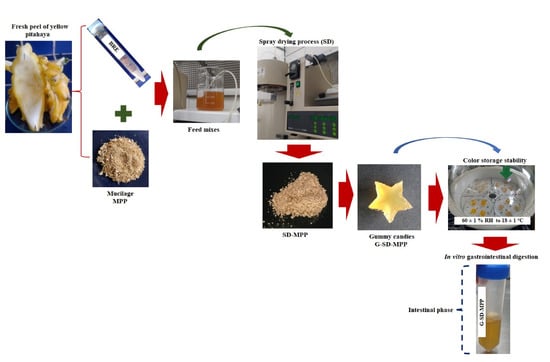
2.3. Extraction of Betaxanthin Pigment and Mucilage from Pitahaya Peel
2.4. Spray-Drying Microencapsulation of Pitahaya Peel Betaxanthins
2.5. Physicochemical Characterization of Betaxanthin Microencapsulates
2.5.1. Betaxanthins Content and Antioxidant Activity
2.5.2. Fourier Transform Infrared Spectroscopy (FTIR) and Zeta Potential
2.5.3. Scanning Electron Microscopy and Particle Size/Distribution
2.5.4. Thermal Behavior
2.6. Application of Betaxanthin Microcapsules for Preparation of Gummies
2.7. Gummy Candy Characterization
2.7.1. Total Dietary Fiber Content
2.7.2. Texture Analysis
2.7.3. In Vitro Evaluation of Gastrointestinal Digestion
2.7.4. Color Stability Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Spray-Drying Microcapsules’ Characterization
3.1.1. Betaxanthin Content and Antioxidant Capacity
3.1.2. FTIR Spectra of Microcapsules SD-MPP and SD-MD
3.1.3. Particle Size, Polydispersity Index, and Zeta Potential of Microcapsules SD-MPP and SD-MD
3.1.4. Microcapsule Morphology by Scanning Electron Microscopy (SEM)
3.1.5. Thermal Behavior
3.2. Characterization of Gummies Formulated with Pitahaya Peel Betaxanthin Microcapsules
3.3. Gummy Candies Stability
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Echegaray, N.; Guzel, N.; Kumar, M.; Guzel, M.; Hassoun, A.; Lorenzo, J.M. Recent advancements in natural colorants and their application as coloring in food and in intelligent food packaging. Food Chem. 2023, 404, 134453. [Google Scholar] [CrossRef]
- Cassani, L.; Marcovich, N.E.; Gomez-Zavaglia, A. Valorization of fruit and vegetables agro-wastes for the sustainable production of carotenoid-based colorants with enhanced bioavailability. Food Res. Int. 2022, 152, 110924. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Spray-Drying. Microencapsulation of Andean Blueberry (Vaccinium meridionale Sw.) Anthocyanins Using Prickly Pear (Opuntia ficus indica L.) Peel Mucilage or Gum Arabic: A Comparative Study. Foods 2023, 12, 1811. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Pu, Y.; Chen, L.; Cao, J.; Jiang, W. Development and characterization of a novel active and intelligent film based on pectin and betacyanins from peel waste of pitaya (Hylocereus undatus). Food Chem. 2023, 404, 134444. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Aguilar, D.M.; López-Martínez, J.M.; Hernández-Brenes, C.; Gutiérrez-Uribe, J.A.; Welti-Chanes, J. Dietary fiber, phytochemical composition and antioxidant activity of Mexican commercial varieties of cactus pear. J. Food Compost. Anal. 2015, 41, 66–73. [Google Scholar] [CrossRef]
- Carreon-Hidalgo, J.P.; Franco-Vasquez, D.C.; Gomez-Linton, D.R.; Perez-Flores, L.J. Betalain plant sources, biosynthesis, extraction, stability enhancement methods, bioactivity, and applications. Food Res. Int. 2022, 151, 110821. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mena, A.; Ochoa-Martínez, L.A.; Gonzalez-Herrera, S.M.; Rutiaga-Quinones, O.M.; Gonzalez-Laredo, R.F.; Olmedilla-Alonso, B. Natural pigments of plant origin: Classification, extraction and application in foods. Food Chem. 2023, 398, 133908. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; Jimenez-Atienzar, M.; Cabanes, J.; García-Carmona, F.; Escribano, J. Stabilization of the bioactive pigment of Opuntia fruits through maltodextrin encapsulation. J. Agric. Food Chem. 2010, 58, 10646–10652. [Google Scholar] [CrossRef]
- Fernandez-Lopez, J.A.; Roca, M.J.; Angosto, J.M.; Obon, J.M. Betaxanthin-rich extract from cactus pear fruits as yellow water-soluble colorant with potential application in foods. Plant Foods Hum. Nutr. 2018, 73, 146–153. [Google Scholar] [CrossRef]
- Otalora, M.C.; Carriazo, J.G.; Osorio, C.; Nazareno, M.A. Encapsulation of cactus (Opuntia megacantha) betaxanthins by ionic gelation and spray drying: A comparative study. Food Res. Int. 2018, 111, 423–430. [Google Scholar] [CrossRef]
- Carmona, J.C.; Robert, P.; Vergara, C.; Saenz, C. Microparticles of yellow-orange cactus pear pulp (Opuntia ficus-indica) with cladode mucilage and maltodextrin as a food coloring in yogurt. LWT Food Sci. Technol. 2021, 138, 110672. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Núñez-Ramírez, D.M.; Calderas, F.; González-Laredo, R.F.; Minjares-Fuentes, R.; Valadez-García, M.A.; Bernad-Bernad, M.J.; Manero, O. Microencapsulation of gallic acid by spray drying with aloe vera mucilage (Aloe barbadensis miller) as wall material. Ind. Crop. Prod. 2019, 138, 111461. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Extraction and Physicochemical Characterization of Dried Powder Mucilage from Opuntia ficus-indica Cladodes and Aloe Vera Leaves: A Comparative Study. Polymers 2021, 13, 1689. [Google Scholar] [CrossRef] [PubMed]
- De Campo, C.; Dick, M.; dos Santos, P.P.; Costa, T.M.H.; Paese, K.; Guterres, S.S.; Rios, A.O.; Flôres, S.H. Zeaxanthin nanoencapsulation with Opuntia monacantha mucilage as structuring material: Characterization and stability evaluation under different temperatures. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 410–421. [Google Scholar] [CrossRef]
- Soto-Castro, D.; Gutiérrez, M.G.; León-Martínez, F.; Santiago-García, P.A.; Aragón-Lucero, I.; Antonio-Antonio, F. Spray drying microencapsulation of betalain rich extracts from Escontria chiotilla and Stenocereus queretaroensis fruits using cactus mucilage. Food Chem. 2019, 272, 715–722. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Spray-Drying Microencapsulation of Pink Guava (Psidium guajava) Carotenoids Using Mucilage from Opuntia ficus-indica Cladodes and Aloe Vera Leaves as Encapsulating Materials. Polymers 2022, 14, 310. [Google Scholar] [CrossRef] [PubMed]
- Medina-Torres, L.; García-Cruz, E.E.; Calderas, F.; González Laredo, R.F.; Sánchez-Olivares, G.; Gallegos-Infante, J.A.; Rocha-Guzmán, N.E.; Rodríguez-Ramírez, J. Microencapsulation by spray drying of gallic acid with nopal mucilage (Opuntia ficus indica). LWT—Food Sci. Technol. 2013, 50, 642–650. [Google Scholar] [CrossRef]
- Tabio-García, D.; Paraguay-Delgado, F.; Lardizabal Gutiérrez, D.; Quintero-Ramos, A.; Meléndez-Pizarro, C.O.; Ochoa-Martínez, L.A.; Sánchez-Madrigal, M.A.; Ruiz-Gutiérrez, M.G.; Espinoza-Hicks, J.C. Effectiveness of Opuntia ficus-indica mucilage as a carrier agent in microencapsulation of bioactive compounds of Amaranthus hypochondriacus var. Nutrisol. Food Biosci. 2023, 52, 102368. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Mucilage from Yellow Pitahaya (Selenicereus megalanthus) Fruit Peel: Extraction, Proximal Analysis, and Molecular Characterization. Molecules 2023, 28, 786. [Google Scholar] [CrossRef]
- Gunes, R.; Palabiyik, I.; Konar, N.; Said Toker, O. Soft confectionery products: Quality parameters, interactions with processing and ingredients. Food Chem. 2022, 385, 132735. [Google Scholar] [CrossRef]
- Rodríguez-Sanchez, J.A.; Cruz y Victoria, M.T.; Barragan-Huerta, B.E. Betaxanthins and antioxidant capacity in Stenocereus pruinosus: Stability and use in food. Food Res. Int. 2017, 91, 63–71. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Natural colorants from plant pigments and their encapsulation: An emerging window for the food industry. LWT—Food Sci. Technol. 2022, 153, 112527. [Google Scholar] [CrossRef]
- Fathordoobady, F.; Mirhosseini, H.; Salamat, J.; Abd Manap, M.Y. Effect of Solvent Type and Ratio on Betacyanins and Antioxidant Activity of Extracts from Hylocereus polyrhizus Flesh and Peel by Supercritical Fluid Extraction and Solvent Extraction. Food Chem. 2016, 202, 70–80. [Google Scholar] [CrossRef]
- Amjadia, S.; Ghorbani, M.; Hamed Hamishehkar, H.; Roufegarinejad, L. Improvement in the stability of betanin by liposomal nanocarriers: Its application in gummy candy as a food model. Food Chem. 2018, 256, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Charoen, R. Development of Antioxidant Gummy Jelly Candy Supplemented with Psidium guajava Leaf Extract. Int. J. Appl. Sci. Technol. 2015, 8, 145–151. [Google Scholar] [CrossRef]
- Cunniff, P. Enzymatic-gravimetric method. In Official Methods of Analysis of AOAC International, 16th ed.; AOAC: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Pacheco, C.; González, E.; Robert, P.; Parada, J. Retention and pre-colon bioaccessibility of oleuropein in starchy food matrices, and the effect of microencapsulation by using inulin. J. Funct. Foods 2018, 41, 112–117. [Google Scholar] [CrossRef]
- Us-Medina, U.; Julio, L.M.; Segura-Campos, M.R.; Ixtaina, V.Y.; Tomas, M.C. Development and characterization of spray-dried chia oil microcapsules using by-products from chia as wall material. Powder Technol. 2018, 334, 1–8. [Google Scholar] [CrossRef]
- de Freitas Santos, P.; Rubio Vieira, F.T.; Palazzolli da Silva, M.; Siva Pinho, L.; Favaro-Trindade, C.S. Microencapsulation of carotenoid-rich materials: A review. Food Res. Int. 2021, 147, 110571. [Google Scholar] [CrossRef]
- Antigo, J.L.D.; Stafussa, A.P.; de Cassia Bergamasco, R.; Madrona, G.S. Chia seed mucilage as a potential encapsulating agent of a natural food dye. J. Food Eng. 2020, 285, 110101. [Google Scholar] [CrossRef]
- Halloub, A.; Raji, M.; Essabir, H.; Nekhlaoui, S.; Bensalah, M.-O.; Bouhfid, R.; el kacem Qaiss, A. Stable smart packaging betalain-based from red prickly pear covalently linked into cellulose/alginate blend films. Int. J. Biol. Macromol. 2023, 234, 123764. [Google Scholar] [CrossRef]
- Calva-Estrada, S.J.; Jiménez-Fernández, M.; Lugo-Cervantes, E. Betalains and their applications in food: The current state of processing, stability and future opportunities in the industry. Food Chem. Mol. Sci. 2022, 4, 100089. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef] [Green Version]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain stability and degradationstructural and chromatic aspects. J. Food Sci. 2006, 71, 41–50. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Núñez-Ramírez, D.M.; Calderas, F.; Bernad-Bernad, M.J.; Gracia-Mora, J.; Rodríguez-Ramírez, J.; González-Laredo, R.F.; Gallegos-Infante, J.A.; Manero, O. Curcumin encapsulation by spray drying using Aloe vera mucilage as encapsulating agent. J. Food Process Eng. 2018, 42, e12972. [Google Scholar] [CrossRef]
- Gheribi, R.; Habibi, Y.; Khwaldia, K. Prickly pear peels as a valuable resource of added-value polysaccharide: Study of structural, functional and film forming properties. Int. J. Biol. Macromol. 2019, 126, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Utpott, M.; Queiroz Assis, R.; Pagno, C.H.; Pereira Krigger, S.; Rodrigues, E.; de Oliveira Rios, A.; Flôres, S.H. Evaluation of the Use of Industrial Wastes on the Encapsulation of Betalains Extracted from Red Pitaya Pulp (Hylocereus polyrhizus) by Spray Drying: Powder Stability and Application. Food Bioprocess Technol. 2020, 13, 1940–1953. [Google Scholar] [CrossRef]
- Bayar, N.; Kriaa, M.; Kammoun, R. Extraction and characterization of three polysaccharides extracted from Opuntia ficus indica cladodes. Int. J. Biol. Macromol. 2016, 92, 441–450. [Google Scholar] [CrossRef]
- Miguel, M.G. Betalains in some species of the amaranthaceae family: A review. Antioxidants 2018, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Robert, P.; Torres, V.; García, P.; Vergara, C.; Saenz, C. The encapsulation of purple cactus pear (Opuntia ficus-indica) pulp by using polysaccharide-proteins as encapsulating agents. LWT—Food Sci. Technol. 2015, 60, 1039–1045. [Google Scholar] [CrossRef]
- Monge Neto, A.A.; Fonseca Tomazini, L.; Gouveia Mizuta, A.; Gomes Correa, R.C.; Scaramal Madrona, G.; Faria de Moraes, F.; Peralta, R.M. Direct microencapsulation of an annatto extract by precipitation of psyllium husk mucilage polysaccharides. Food Hydrocoll. 2021, 112, 106333. [Google Scholar] [CrossRef]
- Alves, E.S.; Ripke Ferreira, C.S.; Souza, P.R.; Silva Bruni, A.R.; Campos Castro, M.; Bruno Figueiredo Saqueti, H.; Oliveira Santos, O.; Scaramal Madrona, G.; Vergilio Visentainer, J. Freeze-dried human milk microcapsules using gum arabic and maltodextrin: An approach to improving solubility. Int. J. Biol. Macromol. 2023, 238, 124100. [Google Scholar] [CrossRef] [PubMed]
- Otálora, M.C.; Carriazo, J.G.; Iturriaga, L.; Nazareno, M.A.; Osorio, C. Microencapsulation of betalains obtained from cactus fruit (Opuntia ficus-indica) by spray drying using cactus cladode mucilage and maltodextrin as encapsulating agents. Food Chem. 2015, 187, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.M.; Oliveira Müller, C.M.; Salvador Ferreira, S.R. Photostability and characterization of spray-dried maltodextrin powders loaded with Sida rhombifolia extract. Biocatal. Agric. Biotechnol. 2020, 27, 101716. [Google Scholar] [CrossRef]
- Fernandes, R.V.; Borges, S.V.; Botrel, D.A. Gum Arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carbohydr. Polym. 2014, 101, 524–532. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Liu, L.; Li, Y.; Qi, B.; Jiang, L. Preparation of spray-dried soybean oil body microcapsules using maltodextrin: Effects of dextrose equivalence. LWT—Food Sci. Technol. 2020, 154, 112874. [Google Scholar] [CrossRef]
- Santiago-Adame, R.; Medina-Torres, L.; Gallegos-Infante, J.A.; Calderas, F.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Ochoa-Martínez, L.A.; Bernad-Bernad, M.J. Spray drying-microencapsulation of cinnamon infusions (Cinnamomum zeylanicum) with maltodextrin. LWT—Food Sci. Technol. 2015, 64, 571–577. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.-H.; Qiao, J.; Qu, W.; Wang, M.-S.; Gao, X.; Zhang, C.; Brennan, C.S.; Qi, X. Improvement of betalains stability extracted from red dragon fruit peel by ultrasound-assisted microencapsulation with maltodextrin. Ultrason. Sonochem. 2022, 82, 105897. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Chang, Y.H. Microencapsulation of a maca leaf polyphenol extract in mixture of maltodextrin and neutral polysaccharides extracted from maca roots. Int. J. Biol. Macromol. 2020, 150, 546–558. [Google Scholar] [CrossRef]
- Kwak, H.-S.; Al Mijan, M.; Ganesan, P. Application of nanomaterials, nano-and microencapsulation to milk and dairy products. Nano- Microencapsul. Foods 2014, 1, 273–300. [Google Scholar] [CrossRef]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Muzaffar, K.; Kumar, P. Parameter optimization for spray drying of tamarind pulp using response surface methodology. Powder Technol. 2015, 279, 179–184. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Anthocyanin stability and antioxidant activity of spray-dried açai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Res. Int. 2010, 43, 907–914. [Google Scholar] [CrossRef]
- Negrao-Murakami, A.N.; Nunes, G.L.; Pinto, S.S.; Murakami, F.S.; Amante, E.R.; Cunha Petrus, J.C.; Prudencio, E.S.; Amboni, R.D.M.C. Influence of DE-value of maltodextrin on the physicochemical properties, antioxidant activity, and storage stability of spray dried concentrated mate (Ilex paraguariensis A. St. Hil.). LWT—Food Sci. Technol. 2017, 79, 561–567. [Google Scholar] [CrossRef]
- Molaveisi, M.; Noghabi, M.S.; Parastouei, K.; Taheri, R.A. Fate of nanophytosomes containing bioactive compounds of Echinacea extract in an acidic food beverage. Food Struct. 2021, 27, 100177. [Google Scholar] [CrossRef]
- Parvez, S.; Wani, I.A.; Masoodi, F.A. Nanoencapsulation of green tea extract using maltodextrin and its characterisation. Food Chem. 2022, 384, 132579. [Google Scholar] [CrossRef]
- Jang, Y.; Koh, E. Characterisation and storage stability of aronia anthocyanins encapsulated with combinations of maltodextrin with carboxymethyl cellulose, gum Arabic, and xanthan gum. Food Chem. 2023, 405, 135002. [Google Scholar] [CrossRef]
- Aberkane, L.; Roudaut, G.; Saurel, R. Encapsulation and oxidative stability of PUFA-rich oil microencapsulated by spray drying using pea protein and pectin. Food Bioprocess Technol. 2014, 7, 1505–1517. [Google Scholar] [CrossRef]
- Mestry, A.P.; Mujumdar, A.S.; Thorat, B.N. Optimization of spray drying of an innovative functional food: Fermented mixed juice of carrot and watermelon. Dry Technol. 2011, 29, 1121–1131. [Google Scholar] [CrossRef]
- Osorio, C.; Acevedo, B.; Hillebrand, S.; Carriazo, J.; Winterhalter, P.; Morales, A.L. Microencapsulation by spray-drying of anthocyanin pigments from Corozo (Bactris guineensis) fruit. J. Agric. Food Chem. 2010, 58, 6977–6985. [Google Scholar] [CrossRef]
- Cortes-Camargo, S.; Acuña-Avila, P.E.; Rodriguez-Huezo, M.E.; Roman-Guerrero, A.; Varela-Guerrero, V.; Perez-Alonso, C. Effect of chia mucilage addition on oxidation and release kinetics of lemon essential oil microencapsulated using mesquite gum—Chia mucilage mixtures. Food Res. Int. 2019, 116, 1010–1019. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation efficiency of food flavours and oils during spray drying. Dry. Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Janiszewsk, E. Microencapsulated beetroot juice as a potential source of betalain. Powder Technol. 2014, 264, 190–196. [Google Scholar] [CrossRef]
- Risch, S.J.; Reineccius, G.A. Encapsulation and controlled release of food ingredients. In ACS symposium Series; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Carpena, M.; Cassani, L.; Gomez-Zavaglia, A.; Garcia-Perez, P.; Seyyedi-Mansour, S.; Cao, H.; Simal-Gandara, J.; Prieto, M.A. Application of fermentation for the valorization of residues from Cactaceae family. Food Chem. 2023, 410, 135369. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Arroyo, A.; Schweiggert, R.M.; Pineda-Castro, M.L.; Sramek, M.; Kohlus, R.; Carle, P.; Esquivel, R. Characterization of cell wall polysaccharides of purple pitaya (Hylocereus sp.) pericarp. Food Hydrocoll. 2014, 35, 557–564. [Google Scholar] [CrossRef]
- Pan, L.-H.; Wu, C.-L.; Luo, S.-Z.; Luo, J.-P.; Zheng, Z.; Jiang, S.-T.; Zhao, Y.-Y.; Zhong, X.-Y. Preparation and characteristics of sucrose-resistant emulsions and their application in soft candies with low sugar and high lutein contents and strong antioxidant activity. Food Hydrocoll. 2022, 129, 107619. [Google Scholar] [CrossRef]
- Constantino, A.B.T.; Garcia-Rojas, E.E. Microencapsulation of beta-carotene by complex coacervation using amaranth carboxymethyl starch and lactoferrin for application in gummy candies. Food Hydrocoll. 2023, 139, 108488. [Google Scholar] [CrossRef]
- Hani, N.M.; Romli, S.R.; Ahmad, M. Influences of red pitaya fruit puree and gelling agents on the physico-mechanical properties and quality changes of gummy confections. Int. J. Food Sci. Technol. 2015, 50, 331–339. [Google Scholar] [CrossRef]
- Mutlua, C.; Tontula, S.A.; Erbaşa, M. Production of a minimally processed jelly candy for children using honey instead of sugar. LWT—Food Sci. Technol. 2018, 93, 499–505. [Google Scholar] [CrossRef]
- Fredes, C.; Osorio, M.J.; Parada, J.; Robert, P. Stability and bioaccessibility of anthocyanins from maqui (Aristotelia chilensis [Mol.] Stuntz) juice microparticles. LWT—Food Sci. Technol. 2018, 91, 549–556. [Google Scholar] [CrossRef]
- Grenha, A.; Guerreiro, F.; Lourenço, J.P.; Lopes, J.A.; Cámara-Martos, F. Microencapsulation of selenium by spray-drying as a tool to improve bioaccessibility in food matrix. Food Chem. 2023, 402, 134463. [Google Scholar] [CrossRef]
- Cakmak, H.; Ilyasoglu-Buyukkestelli, H.; Sogut, E.; Hazal Ozyurt, V.; Gumus-Bonacina, C.E.; Sebnem Simsek, S. A review on recent advances of plant mucilages and their applications in food industry: Extraction, functional properties and health benefits. Food Hydrocoll. Health 2023, 3, 100131. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Zou, L.; Chen, L.; Ahmed, Y.; Al Bishri, W.; McClements, D.J. Encapsulation of curcumin in polysaccharide-based hydrogel beads: Impact of bead type on lipid digestion and curcumin bioaccessibility. Food Hydrocoll. 2016, 58, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Kaimainen, M.; Laaksonen, O.; Järvenpää, E.; Sandell, M.; Huopalahti, R. Consumer acceptance and stability of spray dried betanin in model juices. Food Chem. 2015, 187, 398–406. [Google Scholar] [CrossRef]
- Dias, S.; Castanheira, E.M.S.; Fortes, A.G.; Pereira, D.M.; Gonçalves, M.S.T. Natural pigments of anthocyanin and betalain for coloring soy-based yogurt alternative. Foods 2020, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I. Stabilization of betalains: A review. Food Chem. 2016, 197, 1280–1285. [Google Scholar] [CrossRef]








| Parameter | BRE | SD-MPP | SD-MD |
|---|---|---|---|
| PY (%) | 25.2 | 50.4 | |
| BC 1 | 0.1058 | 0.087 | 0.085 |
| ORAC 2 | 1298.49 ± 160.82 a | 868.68 ± 114.23 b | 366.59 ± 47.31 c |
| Parameter 1 | SD-MPP | SD-MD |
|---|---|---|
| PS (in µm) | 65.01 ± 3.32 a | 1.24 ± 0.16 b |
| PDI | 2.58 ± 0.07 a | 0.34 ± 0.00 b |
| Zeta potential | −30.94 ± 0.74 a | −25.34 ± 2.60 b |
| Parameter | G-SD-MPP | G-SD-MD | G-BRE |
|---|---|---|---|
| TDFC 1 | 0.82 | 0.26 | 0.56 |
| Hardness (g) | 177.3 | 346.6 | 531.6 |
| Adhesiveness (g·s) | - | −101.1 | −19.8 |
| Springiness (mm) | 0.970 | 0.970 | 0.908 |
| Cohesiveness (-) | 450.9 | 0.914 | 0.661 |
| Gumminess (g) | 151.0 | 310.8 | 268.9 |
| Chewiness (g) | 147.1 | 299.6 | 233.5 |
| AAPH• Inhibition (%) 2 | 347.3 | 528.2 | 155.1 |
| Sample 1 | Time (in Days) | ΔE* | ||
|---|---|---|---|---|
| G-SD-MPP | 0 | 531.20 ± 73.02 c | 76.20 ± 1.54 b | 9.94 ± 2.07 c |
| 30 | 770.27 ± 21.71 a | 80.68 ± 0.14 a | ||
| G-SD-MD | 0 | 343.05 ± 9.36 d | 82.58 ± 0.26 a | 10.69 ± 1.03 b |
| 30 | 115.01 ± 22.41 f | 10.69 ± 1.03 d | ||
| G-BRE | 0 | 676.51 ± 49.48 b | 76.70 ± 0.47 b | 17.05 ± 0.72 a |
| 30 | 291.43 ± 24.81 e | 17.05 ± 0.72 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Microencapsulation of Betaxanthin Pigments from Pitahaya (Hylocereus megalanthus) By-Products: Characterization, Food Application, Stability, and In Vitro Gastrointestinal Digestion. Foods 2023, 12, 2700. https://doi.org/10.3390/foods12142700
Otálora MC, Wilches-Torres A, Gómez Castaño JA. Microencapsulation of Betaxanthin Pigments from Pitahaya (Hylocereus megalanthus) By-Products: Characterization, Food Application, Stability, and In Vitro Gastrointestinal Digestion. Foods. 2023; 12(14):2700. https://doi.org/10.3390/foods12142700
Chicago/Turabian StyleOtálora, María Carolina, Andrea Wilches-Torres, and Jovanny A. Gómez Castaño. 2023. "Microencapsulation of Betaxanthin Pigments from Pitahaya (Hylocereus megalanthus) By-Products: Characterization, Food Application, Stability, and In Vitro Gastrointestinal Digestion" Foods 12, no. 14: 2700. https://doi.org/10.3390/foods12142700