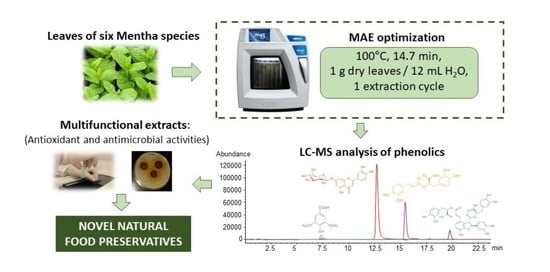
Optimization of a Green Microwave-Assisted Extraction Method to Obtain Multifunctional Extracts of Mentha sp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Standards
2.2. Microwave-Assisted Extraction (MAE)
2.3. Liquid Chromatography–Mass Spectrometry (LC-MS) Analysis
2.4. Total Phenolic Content (TPC)
2.5. Antioxidant Activity (DPPH Assay)
2.6. Antimicrobial Activity
2.7. Statistical Analysis
3. Results
3.1. Optimization of an MAE Method to Provide Multifunctional Extracts of Mentha sp.
3.1.1. Selection of MAE Solvent and s/V Ratio
3.1.2. Optimization of MAE Operating Conditions
3.2. Application of the Optimized MAE Method to Different Mentha Species
3.2.1. LC-MS Characterization
3.2.2. Total Phenolic Content and Antioxidant Activity
3.2.3. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.; Restani, P.; Biella, S.; Di Lorenzo, C. Botanicals in functional foods and food supplements: Tradition, efficacy and regulatory aspects. Appl. Sci. 2020, 10, 2387. [Google Scholar] [CrossRef]
- Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilar, C.N.; et al. Functional importance of bioactive compounds of foods with potential health benefits: A review on recent trends. Food Biosci. 2021, 43, 101320. [Google Scholar] [CrossRef]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Insecticidal properties of Mentha species: A review. Ind. Crops Prod. 2011, 34, 802–817. [Google Scholar] [CrossRef]
- Kimbaris, A.C.; González-Coloma, A.; Andrés, M.F.; Vidali, V.P.; Plissiou, M.G.; Santana-Méridas, O. Biocidal compounds from Mentha sp. essential oils and their structure-activity relationships. Chem. Biodiv. 2017, 14, e1600270. [Google Scholar] [CrossRef] [PubMed]
- Sabo, V.A.; Knezevic, P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind. Crops Prod. 2019, 132, 413–429. [Google Scholar] [CrossRef]
- Stirk, W.A.; van Staden, J. Bioprospecting for bioactive compounds in microalgae: Antimicrobial compounds. Biotech. Adv. 2022, 59, 107977. [Google Scholar] [CrossRef]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)—Prospects and challenges. Biocontrol Sci. Technol. 2019, 29, 207–228. [Google Scholar] [CrossRef]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Lajayer, B.A.; Hadian, J.; Astatkie, T. Applications of Essential Oils and Plant Extracts in different industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef]
- Božović, M.; Pirolli, A.; Ragno, R. Mentha suaveolens Ehrh. (Lamiaceae) essential oil and its main constituent piperitenone oxide: Biological activities and chemistry. Molecules 2015, 20, 8605–8633. [Google Scholar] [CrossRef]
- Gulluce, M.; Sahin, F.; Sokmen, M.; Ozer, H.; Daferera, D.; Sokmen, A.; Polissiou, M.; Adiguzel, A.; Ozkan, H. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. ssp. Longifolia. Food Chem. 2007, 103, 1449–1456. [Google Scholar] [CrossRef]
- Riahi, L.; Elferchichi, M.; Ghazghazi, H.; Jebali, J.; Ziadi, S.; Aouadhi, C.; Chograni, H.; Zaouali, Y.; Zoghlami, N.; Mliki, A. Phytochemistry, antioxidant and antimicrobial activities of the essential oils of Mentha rotundifolia L. in Tunisia. Ind. Crops Prod. 2013, 49, 883–889. [Google Scholar] [CrossRef]
- Thach, L.N.; Nhung, T.H.; My, V.T.N.; Tran, H.A. The new rich source of rotundifolone: Mentha aquatica Linn. var. crispa oil from microwave-assisted hydrodistillation. J. Essent. Oil Res. 2013, 25, 39–43. [Google Scholar] [CrossRef]
- Petrakis, E.A.; Kimbaris, A.C.; Perdikis, D.C.; Lykouressis, D.P.; Tarantilis, P.A.; Polissiou, M.G. Responses of Myzus persicae (Sulzer) to three Lamiaceae essential oils obtained by microwave-assisted and conventional hydrodistillation. Ind. Crops Prod. 2013, 62, 272–279. [Google Scholar] [CrossRef]
- Costa, S.S.; Gariepy, Y.; Rocha, S.C.S.; Raghavan, V. Microwave extraction of mint essential oil—Temperature calibration for the oven. J. Food Eng. 2014, 126, 1–6. [Google Scholar] [CrossRef]
- Gavahian, M.; Farahnaky, A.; Farhoosh, R.; Javidnia, K.; Shahidi, F. Extraction of essential oils from Mentha piperita using advanced techniques: Microwave versus ohmic assisted hydrodistillation. Food Bioprod. Proc. 2015, 94, 50–58. [Google Scholar] [CrossRef]
- Mohammadhosseini, M.; Venditti, A.; Mahdavi, B. Characterization of essential oils and volatiles from the aerial parts of Mentha pulegium L. (Lamiaceae) using microwave-assisted hydrodistillation (MAHD) and headspace solid phase microextraction (HS-SPME) in combination with GC-MS. Nat. Prod. Res. 2023, 37, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Rado, E. Matrix solid-phase dispersion (MSPD) in chromatographic analysis of essential oils in herbs. J. Pharm. Biomed. Anal. 2010, 52, 79–85. [Google Scholar] [CrossRef]
- Ćavar Zeljković, S.; Šišková, J.; Komzáková, K.; De Diego, N.; Kaffková, K.; Tarkowski, P. Phenolic compounds and biological activity of selected Mentha species. Plants 2021, 10, 550. [Google Scholar] [CrossRef]
- Roshanpour, S.; Tavakoli, J.; Beigmohammadi, F.; Alaei, S.; Khaneghah, A.M. Extraction of phenol compound from Mentha piperita by ultrasonic waves based on a response surface methodology. Food Sci. Nutr. 2023, 11, 613–626. [Google Scholar] [CrossRef]
- Dai, J.; Orsat, V.; Raghavan, G.S.V.; Yaylayan, V. Investigation of various factors for the extraction of peppermint (Mentha piperita L.) leaves. J. Food Eng. 2010, 96, 540–543. [Google Scholar] [CrossRef]
- Nejati-Yazdinejad, M.; Soozangar, A. HPLC method for evaluation of antioxidant activity of Mentha pulegium leaves extract. Res. J. Chem. Environ. 2013, 17, 22–26. [Google Scholar]
- García-Sarrió, M.J.; Sanz, M.L.; Sanz, J.; González-Coloma, A.; Soria, A.C. A new method for microwave assisted ethanolic extraction of Mentha rotundifolia bioactive terpenoids. Electrophoresis 2018, 39, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Pavlić, B.; Kaplan, M.; Bera, O.; Olgun, E.O.; Canli, O.; Milosavljević, N.; Antić, B.; Zeković, Z. Microwave-assisted extraction of peppermint polyphenols—Artificial neural networks approach. Food Bioprod. Proc. 2019, 118, 258–269. [Google Scholar] [CrossRef]
- Pavlić, B.; Teslić, N.; Zengin, G.; Đurović, S.; Rakić, D.; Cvetanović, A.; Gunes, A.K.; Zeković, Z. Antioxidant and enzyme-inhibitory activity of peppermint extracts and essential oils obtained by conventional and emerging extraction techniques. Food Chem. 2021, 338, 127724. [Google Scholar] [CrossRef]
- Moldovan, R.I.; Oprean, R.; Benedec, D.; Hanganu, D.; Duma, M.; Oniga, I.; Vlase, L. LC-MS analysis, antioxidant and antimicrobial activities for five species of Mentha cultivated in Romania. Dig. J. Nanomat. Biostruct. 2014, 9, 559–566. [Google Scholar]
- Taamalli, A.; Arráez-Román, D.; Abaza, L.; Iswaldi, I.; Ferández-Gutiérrrez, A.; Zarrouk, M.; Segura-Carretero, A. LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015, 26, 320–330. [Google Scholar] [CrossRef]
- Miguel, M.; Barros, L.; Pereira, C.; Calhelha, R.C.; García, P.A.; Castro, M.A.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical characterization and bioactive properties of two aromatic plants: Calendula officinalis L. (flowers) and Mentha cervina L. (leaves). Food Funct. 2016, 7, 2223–2232. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Qanash, H.; Almuhayawi, M.S.; Al Jaouni, S.K.; Bakri, M.M.; Ganash, M.; Salama, H.M.; Selim, S.; Abdelghany, T.M. Molecular interaction studies and phytochemical characterization of Mentha pulegium L. constituents with multiple biological utilities as antioxidant, antimicrobial, anticancer and anti-hemolytic agents. Molecules 2022, 27, 4824. [Google Scholar] [CrossRef]
- Liu, A.H.; Guo, H.; Ye, M.; Lin, Y.H.; Sun, J.H.; Xu, M.; Guo, D.A. Detection, characterization and identification of phenolic acids in Danshen using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. J. Chromatogr. A 2007, 1161, 170–182. [Google Scholar] [CrossRef]
- Nuengchamnong, N.; Krittasilp, K.; Ingkaninan, K. Characterisation of phenolic antioxidants in aqueous extract of Orthosiphon grandiflorus tea by LC–ESI-MS/MS coupled to DPPH assay. Food Chem. 2011, 127, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Tacherfiout, M.; Kherbachi, S.; Kheniche, M.; Mattonai, M.; Degano, I.; Ribechini, E.; Khettal, B. HPLC-DAD and HPLC-ESI-MS-MS profiles of hydroalcoholic extracts of Chamaemelum nobile and Mentha pulegium, and study of their antihemolytic activity against AAPH-induced hemolysis. S. Afr. J. Bot. 2022, 150, 678–690. [Google Scholar] [CrossRef]
- Singelton, V.L.; Orthofer, R.; Lamuela-Raventos, R.R. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Palá-Paúl, J.; Usano-Alemany, J.; Granda, E.; Soria, A.C. Antifungal and antibacterial activity of the essential oil of Chamaecyparis lawsoniana from Spain. Nat. Prod. Commun. 2012, 7, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Soria, A.C.; Ruiz-Aceituno, L.; Ramos, L.; Sanz, M.L. Microwave assisted extraction of polysaccharides. In Polysaccahrides: Bioactivity and Biotechnology; Ramawat, K.G., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 987–1008. [Google Scholar]
- Ruiz-Aceituno, L.; García-Sarrió, M.J.; Alonso-Rodríguez, B.; Ramos, L.; Sanz, M.L. Extraction of bioactive carbohydrates from artichoke (Cynara scolymus L.) external bracts using microwave assisted extraction and pressurized liquid extraction. Food Chem. 2016, 196, 1156–1162. [Google Scholar] [CrossRef]
- Hossain, M.; Rai, D.; Brunton, N.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolics composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef]
- Brahmi, F.; Hauchard, D.; Guendouze, N.; Madani, K.; Kiendrebeogo, M.; Kamagaju, L.; Stévigny, C.; Chibane, M.; Duez, P. Phenolic composition, in vitro antioxidant effects and tyrosinase inhibitory activity of three Algerian Mentha species: M. spicata (L.), M. pulegium (L.) and M. rotundifolia (L.) Huds (Lamiaceae). Ind. Crops Prod. 2015, 74, 722–730. [Google Scholar] [CrossRef]
- Cirlini, M.; Mena, P.; Tassotti, M.; Herrlinger, K.A.; Nieman, K.M.; Dall’Asta, C.; Del Río, D. Phenolic and volatile composition of a dry spearmint (Mentha spicata L.) extract. Molecules 2016, 21, 1007. [Google Scholar] [CrossRef]
- Benedec, D.; Vlase, L.; Oniga, I.; Mot, A.C.; Silghi-Dumitrescu, R.; Hanganu, D.; Tiperciuc, B.; Crisan, G. LC-MS analysis and antioxidant activity of phenolic compounds from two indigenous species of Mentha. Note I. Farmacia 2013, 61, 262–267. [Google Scholar]
- Pereira, O.R.; Cardoso, S. Overview on Mentha and Thymus polyphenols. Curr. Anal. Chem. 2013, 9, 382–396. [Google Scholar] [CrossRef]
- Silva, T.; Oliveira, C.; Borges, F. Caffeic acid derivatives, analogs and applications: A patent review (2009–2013). Exp. Opin. Ther. Pat. 2014, 24, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Mišan, A.C.; Mimica-Dukić, N.M.; Mandić, A.I.; Sakač, M.B.; Milovanovič, I.L.; Sedej, I.J. Development of a rapid resolution HPLC method for the separation and determination of 17 phenolic compounds in crude plant extracts. Cent. Eur. J. Chem. 2011, 9, 133–142. [Google Scholar] [CrossRef]
- Koşar, M.; Dorman, H.J.D.; Başer, K.H.C.; Hiltunen, R. Screening of free radical scavenging compounds in water extracts of Mentha samples using a postcolumn derivatization method. J. Agric. Food Chem. 2004, 52, 5004–5010. [Google Scholar] [CrossRef]
- Proestos, C.; Chorianopoulos, N.; Nychas, J.E.; Komaitis, M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J. Agric. Food Chem. 2005, 53, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Fazal, H.; Ahmad, I.; Abbasi, B.H. Free radical scavenging (DPPH) potential in nine Mentha species. Toxicol. Ind. Health 2011, 28, 83–89. [Google Scholar] [CrossRef]
- Stagos, D.; Portesis, N.; Spanou, C.; Mossialos, D.; Aligiannis, N.; Chaita, E.; Panagoulis, C.; Reri, E.; Skaltsounis, L.; Tsatsakis, A.M. Correlation of total polyphenolic content with antioxidant and antibacterial activity of 24 extracts from Greek domestic Lamiaceae species. Food Chem. Toxicol. 2012, 50, 4115–4124. [Google Scholar] [CrossRef]
- Zekri, N.; Zerkani, H.; Elazzouzi, H.; Zair, T.; El Belghiti Alaoui, M. Extracts of M. pulegium (L.) and M. spicata (L.): Effect of extraction conditions on phenolics and flavonoids contents and their antioxidant power. Egypt. J. Chem. 2021, 64, 1447–1459. [Google Scholar] [CrossRef]
- Krzyzanowska, J.; Janda, B.; Pecio, Ł.; Stochmal, A.; Oleszek, W.; Czubacka, A.; Przybys, M.; Doroszewska, T. Determination of polyphenols in Mentha longifolia and M. piperita field-grown and in vitro plant samples using UPLC-TQ-MS. J. AOAC Int. 2011, 94, 43–50. [Google Scholar] [CrossRef]
- Aldogman, B.; Bilel, H.; Nabil Moustafa, S.M.; Elmassary, K.F.; Ali, H.M.; Qayid Alotaibi, F.; Hamza, M.; Abdelgawad, M.A.; El-Ghora, A.H. Investigation of chemical compositions and biological activities of Mentha suaveolens L. from Saudi Arabia. Molecules 2022, 27, 2949. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Koşar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J. Agric. Food Chem. 2003, 51, 4563–4569. [Google Scholar] [CrossRef]
- Rita, I.; Pereira, C.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C. Mentha spicata L. infusions as sources of antioxidant phenolic compounds: Emerging reserve lots with special harvest requirements. Food Funct. 2016, 7, 4188–4192. [Google Scholar] [CrossRef] [PubMed]
- Politi, M.; Rodrigues, C.L.; Gião, M.S.; Pintado, M.E.; Castro, P.M.L. Antioxidant principles and volatile constituents from the North-Western Iberian mint “Erva-Peixeira”, Mentha cervine. Nat. Prod. Commun. 2008, 3, 2065–2068. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Zengin, G.; Bahadori, S.; Dinparast, L.; Movahhedin, N. Phenolic composition and functional properties of wild mint (Mentha longifolia var. calliantha (Stapf) Briq.). Int. J. Food Prop. 2018, 21, 198–208. [Google Scholar] [CrossRef]
- Yahia, I.B.H.; Zaouali, Y.; Ciavatta, M.L.; Ligresti, A.; Jaouadi, R.; Boussaid, M.; Cutignano, A. Polyphenolic profiling, quantitative assessment and biological activities of Tunisian native Mentha rotundifolia (L.) Huds. Molecules 2019, 24, 2351. [Google Scholar] [CrossRef]
- Singh, S.; Das, S.S.; Singh, G.; Perotti, M.; Schuff, C.; Catalán, C.A.N. In vitro antioxidant potentials and chemistry of essential oils and oleoresins from fresh and sun-dried Mentha longifolia L. J. Essent. Oil Res. 2015, 27, 61–69. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Salgado, J.M.; Domínguez, J.M.; Cortés-Diéguez, S. Comparison of Soxhlet, accelerated solvent and supercritical fluid extraction techniques for volatile (GC–MS and GC/FID) and phenolic compounds (HPLC-ESI/MS/MS) from Lamiaceae species. Phytochem. Anal. 2014, 26, 61–71. [Google Scholar] [CrossRef] [PubMed]
| Experiment | T (°C) | t (min) | RTPC (mg·GAE mL−1) | RDPPH (mg TE mL−1) | RAM (cm) |
|---|---|---|---|---|---|
| 1 | 50 | 17.5 | 4.83 (0.09) * | 6.9 (0.1) | - |
| 2 | 50 | 5 | 5.12 (0.06) | 6.1 (0.3) | - |
| 3 | 50 | 30 | 4.5 (0.4) | 6.8 (0.5) | - |
| 4 | 75 | 5 | 5.45 (0.06) | 8.9 (0.3) | 1.2 (0.1) |
| 5 | 75 | 30 | 5.48 (0.04) | 7.3 (0.4) | 1.1 (0.1) |
| 6 | 100 | 30 | 5.80 (0.08) | 7.1 (0.2) | 1.4 (0.3) |
| 7 | 75 | 17.5 | 5.2 (0.1) | 7.1 (0.3) | 1.7 (0.3) |
| 8 | 100 | 17.5 | 5.58 (0.05) | 8.4 (0.1) | 1.7 (0.3) |
| 9 | 100 | 5 | 5.5 (0.1) | 8.7 (0.7) | 1.6 (0.2) |
| RD | 100 | 14.7 | |||
| Calculated responses | 5.45 | 9.2 | 1.57 | ||
| Experimental responses | 5.26 | 6.18 | 1.70 | ||
| Compound | M. spicata | M. pulegium | M. cervina | M. longifolia | M. rotundifolia | M. suaveolens |
|---|---|---|---|---|---|---|
| 4-Hydroxybenzoic acid | - a | 0.45 (0.09) a* | 4.36 (0.07) b | 3.7 (0.4) b | 0.69 (0.02) a | 12.1 (0.9) c |
| 3-Hydroxy-tyrosol | - a | - a | 0.033 (0.004) c | 0.0243 (0.0004) b | - a | 0.033 (0.005) c |
| p-Coumaric acid | tr a | tr a | tr a | 0.030 (0.001) a | - a | 0.15 (0.06) b |
| Vanillic acid | - a | 0.0122 (0.0003) a,b | 0.03 (0.01) b,c | 0.05 (0.04) c | - a | - a |
| Caffeic acid | 0.020 (0.001) b | - a | tr a | 0.010 (0.004) b | 0.043 (0.008) c | 0.014 (0.001) b |
| Quinic acid | - a | 0.092 (0.009) b | 0.08 (0.01) b | - a | 0.15 (0.02) c | - a |
| trans-Ferulic acid | tr | tr | tr | - | - | - |
| Syringic acid | 0.5 (0.1) a | - a | 4.1 (0.9) c | 0.14 (0.05) a | 1.7 (0.7) b | - a |
| Luteolin | 0.0005 (0.00002) c | 0.0002 (0.0001) a,b | - a | 0.0003 (0.0002) b | tr a | 0.00016 (0.00005) a,b |
| Chlorogenic acid | 0.020 (0.005) d | 0.009 (0.001) c | 0.016 (0.005) d | 0.006 (0.001) b,c | 0.0033 (0.0007) a,b | tr a |
| Rosmarinic acid | 4.4 (0.8) d | 0.15 (0.01) a | 3.2 (0.9) c | 0.22 (0.01) a | 2.5 (0.1) b,c | 1.7 (0.5) b |
| Luteolin-7-O-glucoside | 0.004 (0.001) b | 0.00003 (0.00001) a | - a | - a | 0.002 (0.002) b | - a |
| Salvianolic acid A | - a | 0.019 (0.001) a | 0.4 (0.1) b | - a | 1.2 (0.2) c | - a |
| Salvianolic acid B | 0.66 (0.05) c | 0.0126 (0.0002) a | tr a | 0.0204 (0.0002) a | 0.5 (0.1) b | - a |
| Sagerinic acid | - a | 0.026 (0.001) a | 0.4 (0.1) c | - a | 0.2 (0.1) a,b | 0.3 (0.1) b,c |
| M. spicata | M. suaveolens | M. longifolium | M. pulegium | M. cervina | M. rotundifolia | |||
|---|---|---|---|---|---|---|---|---|
| Growth inhibition halo (cm) | Bactericidal effect | E. coli | 1.00 (0.05) *,a | 1.00 (0.05) a | 1.00 (0.05) a | 1.00 (0.05) a | 1.00 (0.05) a | - |
| S. typhimurium | - | - | - | - | - | - | ||
| S. aureus | 1.35 (0.07) b | - | 1.00 (0.05) a | 1.00 (0.05) a | 1.00 (0.05) a | 1.7 (0.3) b | ||
| Bacteriostatic effect | E. coli | - | 2.4 (0.2) a | - | 2.5 (0.3) a | 2.6 (0.1) a | - | |
| S. aureus | - | - | - | 3.2 (0.2) a | 3.3 (0.3) a | - | ||
| C. albicans | - | - | - | - | 1.7 (0.3) | - | ||
| TPC (mg·GAE·g−1 of dry sample) ** | 48 (1) c | 34.0 (0.2) b | 26.2 (0.7) b | 18.7 (0.1) a | 30.72 (0.07) b | 48.6 (0.6) c | ||
| DPPH (mg·TE·g−1 of dry sample) *** | 120 (9) c | 69 (5) b | 51 (4) a | 55 (2) a | 49 (7) a | 57 (2) a | ||
| IC50 (μg·mL−1) | 99 (6) a | 118 (7) a | 247 (9) c | 359 (6) d | 150 (3) b | 168 (5) b | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Sarrió, M.J.; Sanz, M.L.; Palá-Paúl, J.; Díaz, S.; Soria, A.C. Optimization of a Green Microwave-Assisted Extraction Method to Obtain Multifunctional Extracts of Mentha sp. Foods 2023, 12, 2039. https://doi.org/10.3390/foods12102039
García-Sarrió MJ, Sanz ML, Palá-Paúl J, Díaz S, Soria AC. Optimization of a Green Microwave-Assisted Extraction Method to Obtain Multifunctional Extracts of Mentha sp. Foods. 2023; 12(10):2039. https://doi.org/10.3390/foods12102039
Chicago/Turabian StyleGarcía-Sarrió, María J., María L. Sanz, Jesús Palá-Paúl, Silvia Díaz, and Ana C. Soria. 2023. "Optimization of a Green Microwave-Assisted Extraction Method to Obtain Multifunctional Extracts of Mentha sp." Foods 12, no. 10: 2039. https://doi.org/10.3390/foods12102039