Effects of Storage and Roasting Condition on the Antioxidant Activity of Soybeans with Different Colors of Seed Coat
Abstract
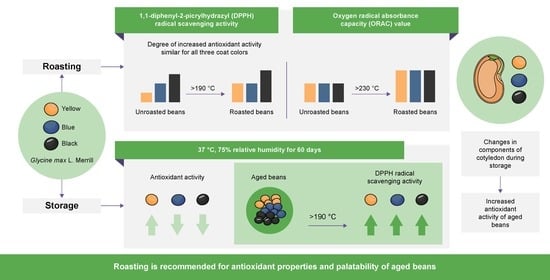
:1. Introduction
2. Materials and Methods
2.1. Soybean Samples
2.2. Storage Conditions
2.3. Roasting Conditions
2.4. Water Content of Roasted Soybean
2.5. Color Measurement
2.6. Preparation for Soybean Extract
2.7. Antioxidant Activity
2.8. Total Phenolic Content
2.9. Assessed the ACE Inhibitory Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Color Characteristic of Roasted Soybean
3.2. Antioxidant Activity and TPC of Soybean
3.3. Color Characteristic of Aged Soybeans
3.4. Antioxidant Activity and TPC due to Roasting in Aged Soybeans
3.5. Antioxidant Activity with or without Seed Coat
3.6. ACE Inhibition Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Troszynska, A.; Estrella, I.; Lopez-Amores, M.L.; Hernandez, T. Antioxidant activity of pea (Pisum sativum L.) seed coat acetone extract. LWT Food Sci. Technol. 2002, 35, 158–164. [Google Scholar] [CrossRef]
- Sushama, A.M.; Rajalakshmi, V.; Sahayog, N.; Jamdar, A.S. Comparative study on antioxidant activity of different varieties of commonly consumed legumes in India. Food Chem. Toxicol. 2011, 49, 2005–2012. [Google Scholar] [CrossRef]
- Takahata, Y.; Ohnishi-Kameyama, M.; Frura, S.; Takahashi, M.; Suda, I. Highly polymerized procyanidins in brown soybean seed coat with a high radical-scavenging activity. J. Agric. Food Chem. 2001, 49, 5843–5847. [Google Scholar] [CrossRef]
- Irene, W.; Elizabeth, W.; Daniel, S.; Clare, K.; Tara, G.; Ann, V.L.; Hendrickx, M. Thermal treatment of common beans (Phaseolus vulgaris L.): Factors determining cooking time and its consequences for sensory and nutritional quality. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1–29. [Google Scholar]
- Hincks, M.J.; Stanley, D.W. Multiple mechanisms of bean hardening. J. Food Sci. Technol. 1986, 21, 731–750. [Google Scholar] [CrossRef]
- Shiga, T.M.; Lajolo, F.M.; Filisetti, T.M. Change in the cell wall polysaccharides during storage and hardening of beans. Food Chem. 2004, 84, 53–64. [Google Scholar] [CrossRef]
- Daniel, M.N.; Peter, K.K.; Claire, M.C.; Stefanie, C.; Anselimo, O.M.; Daniel, N.S.; Marc, E.H. Mechanistic insight into common bean pectic polysaccharide changes during storage, soaking and thermal treatment in relation to the hard-to-cook defect. Food Res. Int. 2016, 81, 39–49. [Google Scholar] [CrossRef]
- Liu, K.; Mc Watters, K.H.; Phillips, R.D. Protein insolubilization and thermal destabilization during storage as related to hard-to-cook defect in cowpeas. J. Agric. Food Chem. 1992, 40, 2483–2487. [Google Scholar] [CrossRef]
- Koriyama, T.; Sato, Y.; Iijima, K.; Kasai, M. Influences of soaking temperature and storage conditions on hardening of soybeans (Glycine max) and red kidney beans (Phaseolus vulgaris). J. Food Sci. 2017, 82, 1546–1555. [Google Scholar] [CrossRef]
- Koriyama, T.; Kasai, M. Effect of pre-soaking treatment on softening and hardening during cooking for storage beans. Food Sci. Technol. Res. 2019, 25, 425–434. [Google Scholar] [CrossRef]
- Jeong, S.M.; Kim, S.Y.; Kim, D.R.; Nam, K.C.; Ahn, D.U.; Lee, S.C. Effect of seed roasting conditions on the antioxidant activity of defatted sesame meal extracts. J. Food Sci. 2004, 69, 377–381. [Google Scholar] [CrossRef]
- Açar, C.; Gökmen, V.; Pellegrini, N.; Fogliano, V. Direct evaluation of the total antioxidant capacity of raw and roasted pulses, nuts and seeds. Eur. Food Res. Technol. 2009, 229, 961–966. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Liu, R.H. Processed sweet corn has higher antioxidant activity. J. Agric. Food Chem. 2002, 50, 4959–4964. [Google Scholar] [CrossRef] [PubMed]
- Rongrong, Z.; Weixi, C.; Baojun, X. Phytochemical profiles of black and yellow soybeans as affected by roasting. Int. J. Food Properities 2017, 20, 3179–3190. [Google Scholar] [CrossRef] [Green Version]
- Žilić, S.; Mogol, B.A.; Akıllıoğlu, G.; Serpen, A.; Delić, N.; Gökmen, V. Effects of extrusion, infrared and microwave processing on Maillard reaction products and phenolic compounds in soybean. J. Sci. Food Agric. 2013, 4, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxdant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cuter, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biology. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Praveen, J.; Lochan, S.; Kshitiz, K.; Vijay, S. Physico-functional and antioxidant properties of sand-roasted chickpea (Cicer arietinum). Food Chem. 2017, 237, 1124–1132. [Google Scholar] [CrossRef]
- Manzocco, L.; Calligaris, S.; Mastrocola, D.; Mocoli, M.C.; Lerici, C.R. Review of non-enzymatic browning and antioxidant capacity in proceeded foods. Trends Food Sci. Technol. 2001, 11, 340–346. [Google Scholar] [CrossRef]
- Hyo, G.K.; Gi, W.K.; Hyein, O.; Se Young, Y.; Young, O.K.; Myung, S.O. Influence of roasting on the antioxidant activity of small black soybean (Glycine max L. Merrill). Food Sci. Technol. 2011, 44, 992–998. [Google Scholar] [CrossRef]
- Hayase, F.; Hirashima, S.; Okamoto, G.; Kato, H. Scavenging of active oxygens by melanoidins. Agric. Biol. Chem. 1989, 53, 3383–3385. [Google Scholar] [CrossRef] [Green Version]
- Saulnier, L.; Marot, C.; Elgorriaga, M.; Bonnin, E.; Thibault, J.-F. Thermal and enzymatic treatments for the release of free ferulic acid from maize bran. Carbohydr. Polym. 2001, 45, 269–275. [Google Scholar] [CrossRef]
- Saio, K.; Arisaka, M. Deterioration of soybean during storage under high moisture and temperature. Nippon Shokuhin Kogyo Gakkaishi. 1978, 25, 451–457. [Google Scholar] [CrossRef]
- Betancur-Ancona, D.; Sosa-Espinoza, T.; Ruiz-Ruiz, J.; Segura-Campos, M.; Chel-Guerr, L. Enzymatic hydrolysis of hard-to-cook bean (Phaseolus vulgaris L.) protein concentrates and its effects on biological and functional properties. Int. J. Food Sci. Technol. 2014, 49, 2–8. [Google Scholar] [CrossRef]
- Machado, C.M.; Ferruzzi, M.G. Impact of the hard-to-cook phenomenon on phenolic antioxidants in dry beans (Phaseolus vulgaris). J. Agric. Food Chem. 2008, 56, 3102–3110. [Google Scholar] [CrossRef]
- Wu, J.; Muir, A.D. Isoflavone content and its potential contribution to the antihypertensive activity in soybean angiotensin converting enzyme inhibitory peptides. J. Agric. Food Chem. 2008, 56, 9899–9904. [Google Scholar] [CrossRef]






| Energy | Water | Protein | Lipid | Carbohydrate 1 | Ash | Na | |
|---|---|---|---|---|---|---|---|
| kcal/100 g | g/100 g | g/100 g | g/100 g | g/100 g | g/100 g | mg/100 g | |
| Yellow soybean | 411 | 13.5 | 32.4 | 16.7 | 32.8 | 4.6 | 2 |
| Blue soybean | 419 | 13.1 | 34.8 | 17.7 | 30 | 4.4 | 1 |
| Black soybean | 417 | 13.6 | 31.2 | 17.9 | 32.8 | 4.5 | 2 |
| Roasting Temperature (℃) | L* | a* | b* | ΔE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yellow soybean | Unroasted | 89.9 | ± | 1.1 | −3.3 | ± | 0.1 | 25.8 | ± | 0.7 | |||
| 150 | 80.4 | ± | 3.2* | 2.9 | ± | 1.8* | 27.8 | ± | 3.3 | 11.5 | ± | 4.0 | |
| 170 | 78.6 | ± | 2.1* | 5.3 | ± | 1.3* | 30.8 | ± | 1.0* | 15.0 | ± | 2.3 | |
| 190 | 65.0 | ± | 4.3* | 8.8 | ± | 0.8** | 32.3 | ± | 2.1* | 28.4 | ± | 4.7 | |
| 210 | 47.0 | ± | 2.0** | 11.2 | ± | 0.6** | 29.9 | ± | 1.9* | 45.5 | ± | 3.1 | |
| 230 | 30.1 | ± | 3.6** | 9.7 | ± | 2.3** | 18.5 | ± | 7.8* | 61.6 | ± | 10.4 | |
| Blue soybean | Unroasted | 78.2 | ± | 5.4 | −5.5 | ± | 3.5 | 25.6 | ± | 2.3 | |||
| 150 | 78.4 | ± | 2.7 | 0.1 | ± | 1.0 | 29.7 | ± | 1.5 | 7.0 | ± | 3.1 | |
| 170 | 72.7 | ± | 3.3 | 5.5 | ± | 0.9 | 34.1 | ± | 0.8 | 14.9 | ± | 1.8 | |
| 190 | 56.5 | ± | 2.8** | 9.9 | ± | 1.4** | 32.0 | ± | 3.5* | 27.3 | ± | 9.4 | |
| 210 | 45.3 | ± | 4.5** | 11.8 | ± | 1.4** | 27.7 | ± | 5.8 | 37.2 | ± | 9.5 | |
| 230 | 28.3 | ± | 4.8** | 8.2 | ± | 3.0** | 12.7 | ± | 7.3* | 53.3 | ± | 12.6 | |
| Black soybean | Unroasted | 76.1 | ± | 5.0 | −1.7 | ± | 1.6 | 21.9 | ± | 1.7 | |||
| 150 | 77.9 | ± | 0.3 | 0.7 | ± | 0.6 | 23.2 | ± | 1.7 | 3.0 | ± | 2.0 | |
| 170 | 70.0 | ± | 1.0* | 5.9 | ± | 0.4* | 30.3 | ± | 1.2* | 14.5 | ± | 2.7 | |
| 190 | 57.2 | ± | 9.2 | 10.0 | ± | 2.4* | 31.5 | ± | 2.2* | 26.5 | ± | 8.0 | |
| 210 | 49.2 | ± | 4.3* | 10.5 | ± | 1.0*** | 28.6 | ± | 2.5* | 32.8 | ± | 6.6 | |
| 230 | 23.3 | ± | 7.2** | 7.8 | ± | 2.1** | 12.1 | ± | 4.5* | 57.2 | ± | 12.1 | |
| ORAC Values | Total Polyphenol Content | L* | ΔE | ||
|---|---|---|---|---|---|
| Yellow soybean | DPPH radical scavenging activity | 0.961 | 0.980 | 0.935 | 0.936 |
| ORAC values | - | 0.932 | 0.915 | 0.911 | |
| Blue soybean | DPPH radical scavenging activity | 0.968 | 0.981 | 0.962 | 0.969 |
| ORAC values | - | 0.977 | 0.895 | 0.905 | |
| Black soybean | DPPH radical scavenging activity | 0.908 | 0.971 | 0.979 | 0.974 |
| ORAC values | - | 0.932 | 0.940 | 0.945 |
| L* | a* | b* | ΔE | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yellow soybean | Control | Unroasted | 89.9 | ± | 1.1 | −3.3 | ± | 0.1 | 25.8 | ± | 0.7 | |||
| Aged | Unroasted | 85.9 | ± | 0.8 | −1.0 | ± | 1.5 | 24.8 | ± | 1.3 | 4.0 | ± | 0.6 | |
| Roasted | 62.8 | ± | 6.9 | 10.2 | ± | 3.4 | 31.2 | ± | 2.1 | 30.8 | ± | 7.1 | ||
| Blue soybean | Control | Unroasted | 78.2 | ± | 5.4 | −5.5 | ± | 3.5 | 25.6 | ± | 2.3 | |||
| Aged | Unroasted | 74.3 | ± | 5.1 | −2.0 | ± | 1.7 | 26.7 | ± | 4.1 | 8.8 | ± | 2.1 | |
| Roasted | 55.7 | ± | 7.1 | 12.4 | ± | 1.7 | 28.8 | ± | 2.8 | 29.0 | ± | 8.0 | ||
| Black soybean | Control | Unroasted | 76.1 | ± | 5.0 | −1.7 | ± | 1.6 | 21.9 | ± | 1.7 | |||
| Aged | Unroasted | 73.5 | ± | 3.3 | 2.4 | ± | 1.8 | 24.8 | ± | 3.4 | 6.6 | ± | 4.3 | |
| Roasted | 55.3 | ± | 3.2 | 12.1 | ± | 1.5 | 28.0 | ± | 3.5 | 25.9 | ± | 7.4 |
| Yellow Soybean | Blue Soybean | Black Soybean | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Aged | Control | Aged | Control | Aged | ||||||||||||||
| ACE inhibitation rate (%) | Untreated | 30.0 | ± | 5.4 a | 59.2 | ± | 2.9 b | 54.2 | ± | 14.2 a | 55.3 | ± | 9.3 a | 57.1 | ± | 8.2 a | 77.3 | ± | 4.4 b |
| Roasted | 41.0 | ± | 10.9 a | 52.4 | ± | 7.8 b | 49.8 | ± | 10.6 a | 41.0 | ± | 7.8 b | 55.1 | ± | 13.7 a | 62.3 | ± | 5.1 a | |
| IC50 (mg/mL) | Untreated | 2.72 | 0.57 | 3.13 | 1.45 | 0.72 | nd | ||||||||||||
| Roasted | 2.73 | 0.82 | 3.24 | 1.68 | 1.90 | 0.65 | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koriyama, T.; Teranaka, K.; Tsuchida, M.; Kasai, M. Effects of Storage and Roasting Condition on the Antioxidant Activity of Soybeans with Different Colors of Seed Coat. Foods 2023, 12, 92. https://doi.org/10.3390/foods12010092
Koriyama T, Teranaka K, Tsuchida M, Kasai M. Effects of Storage and Roasting Condition on the Antioxidant Activity of Soybeans with Different Colors of Seed Coat. Foods. 2023; 12(1):92. https://doi.org/10.3390/foods12010092
Chicago/Turabian StyleKoriyama, Takako, Kiriko Teranaka, Mitose Tsuchida, and Midori Kasai. 2023. "Effects of Storage and Roasting Condition on the Antioxidant Activity of Soybeans with Different Colors of Seed Coat" Foods 12, no. 1: 92. https://doi.org/10.3390/foods12010092