Anticoagulant and Fibrinolytic Properties of Two Heparinoid Compounds Prepared from Shrimp Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Extraction of Heparinoids and Purification from Shrimp Heads
2.4. Disaccharide Composition Analysis
2.5. Methylation Analysis
2.6. NMR Analysis
2.7. Anticoagulant Effects of Heparinoids
2.8. Hemorrhagic Effects of Heparinoids
2.9. Fibrinolytic Activity In Vitro of Heparinoids
2.10. Effect of Heparinoids on Thrombosis and Fibrinolytic Activity In Vivo
2.11. Statistics Analysis
3. Results
3.1. Preparation and Chemical Analysis of Two Heparinoids from Shrimp Heads
3.2. Disaccharide Composition of Two Heparinoids from Shrimp Head
3.3. Methylation Analysis
3.4. NMR Analysis
3.5. Anticoagulant Activity of Heparinoids from Shrimp Head
3.6. Hemorrhagic Effects
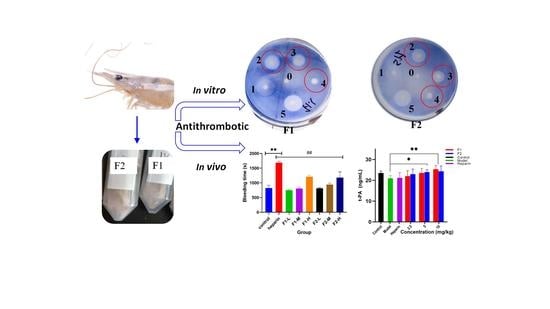
3.7. Fibrinolytic Activity In Vitro
3.8. Effect on Thrombosis and Fibrinolytic Activity In Vivo
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisheries, B.O. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2021. [Google Scholar]
- Nirmal, N.P.; Santivarangkna, C.; Rajput, M.S.; Benjakul, S. Trends in shrimp processing waste utilization: An industrial prospective. Trends Food Sci. Technol. 2020, 103, 20–35. [Google Scholar] [CrossRef]
- Cahú, T.B.; Santos, S.D.; Mendes, A.; Córdula, C.R.; Chavante, S.F.; Carvalho, L.B.; Nader, H.B.; Bezerra, R.S. Recovery of protein, chitin, carotenoids and glycosaminoglycans from Pacific white shrimp (Litopenaeus vannamei) processing waste. Process Biochem. 2012, 47, 570–577. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Wang, Y.; Chen, H.; Liu, H.; Wang, L.; Battino, M.; Yao, X.; Zhu, B.; Du, M. Anticoagulant Dodecapeptide Suppresses Thrombosis In Vivo by Inhibiting the Thrombin Exosite-I Binding Site. J. Agric. Food Chem. 2021, 69, 10920–10931. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Huang, S.; Luo, C.; Wu, Z.; Liang, B.; Huang, H.; Ci, Z.; Zhang, D.; Han, L.; Lin, J. Pharmacological and clinical application of heparin progress: An essential drug for modern medicine. Biomed Pharm. 2021, 139, 111561. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, R.; Vairamani, S.; Shanmugam, A. Glycosaminoglycans from marine clam Meretrix meretrix (Linne.) are an anticoagulant. Prep. Biochem. Biotechnol. 2010, 40, 305–315. [Google Scholar] [CrossRef]
- Gomes, A.M.; Kozlowski, E.O.; Pomin, V.H.; de Barros, C.M.; Zaganeli, J.L.; Pavao, M.S. Unique extracellular matrix heparan sulfate from the bivalve Nodipecten nodosus (Linnaeus, 1758) safely inhibits arterial thrombosis after photochemically induced endothelial lesion. J. Biol. Chem. 2010, 285, 7312–7323. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, C.P.; Paiva, J.F.; Castro, R.A.B.; Chavante, S.F.; Jeske, W.; Fareed, J.; Gorin, P.A.J.; Mendes, A.; Nader, H.B. Structural features and anticoagulant activities of a novel natural low molecular weight heparin from the shrimp Penaeus brasiliensis. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 1999, 1428, 273–283. [Google Scholar] [CrossRef]
- Palhares, L.; Brito, A.S.; de Lima, M.A.; Nader, H.B.; London, J.A.; Barsukov, I.L.; Andrade, G.P.V.; Yates, E.A.; Chavante, S.F. A further unique chondroitin sulfate from the shrimp Litopenaeus vannamei with antithrombin activity that modulates acute inflammation. Carbohydr. Polym. 2019, 222, 115031. [Google Scholar] [CrossRef]
- Chen, J.; Du, Z.; Chen, J.; Jia, X.; Liu, X.; Zhong, S. Preparation, physicochemical properties and anticoagulant activity of heparinoid from shrimp head. Food Sci. 2021, 42, 71–77. [Google Scholar] [CrossRef]
- Carnachan, S.M.; Hinkley, S.F. Heparan Sulfate Identification and Characterisation: Method II. Enzymatic Depolymerisation and SAX-HPLC Analysis to Determine Disaccharide Composition. Bio-Protocal 2017, 7, e2197. [Google Scholar] [CrossRef]
- Sims, I.M.; Carnachan, S.M.; Bell, T.J.; Hinkley, S.F.R. Methylation analysis of polysaccharides: Technical advice. Carbohydr. Polym. 2018, 188, 1–7. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Song, H.; Wang, L.; Peng, H.; Sun, Y.; Ai, C.; Wen, C.; Zhu, B.; Song, S. Structural characterization and SARS-CoV-2 inhibitory activity of a sulfated polysaccharide from Caulerpa lentillifera. Carbohydr. Polym. 2022, 280, 119006. [Google Scholar] [CrossRef] [PubMed]
- CHP. Pharmacopoeia of the People’s Republic of China; People’s Medical Publishing: Beijing, China, 2020; Volume II. [Google Scholar]
- Madeira, J.C.; da Silva, G.V.L.; Batista, J.J.; Saraiva, G.D.; Santos, G.R.C.; Assreuy, A.M.S.; Mourao, P.A.S.; Pereira, M.G. An arabinogalactan-glycoconjugate from Genipa americana leaves present anticoagulant, antiplatelet and antithrombotic effects. Carbohydr. Polym. 2018, 202, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, J.; Li, Y.; Ma, Y.; Wang, W.; Zhong, W.; Li, P.; Du, S. A Novel Fibrinolytic Protein From Pheretima vulgaris: Purification, Identification, Antithrombotic Evaluation, and Mechanisms Investigation. Front. Mol. Biosci. 2021, 8, 772419. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Jia, X.; Chen, J.; Zhou, S.; Chen, J.; Liu, X.; Cao, X.; Zhong, S.; Hong, P. Isolation and Characterization of a Heparin-Like Compound with Potent Anticoagulant and Fibrinolytic Activity from the Clam Coelomactra antiquata. Mar. Drugs. 2019, 18, 6. [Google Scholar] [CrossRef] [Green Version]
- Ma, N.; Liu, X.W.; Yang, Y.J.; Shen, D.S.; Zhao, X.L.; Mohamed, I.; Kong, X.J.; Li, J.Y. Evaluation on antithrombotic effect of aspirin eugenol ester from the view of platelet aggregation, hemorheology, TXB2/6-keto-PGF1alpha and blood biochemistry in rat model. BMC Vet. Res. 2016, 12, 108. [Google Scholar] [CrossRef] [Green Version]
- Andrade, G.P.; Lima, M.A.; de Souza Junior, A.A.; Fareed, J.; Hoppensteadt, D.A.; Santos, E.A.; Chavante, S.F.; Oliveira, F.W.; Rocha, H.A.; Nader, H.B. A heparin-like compound isolated from a marine crab rich in glucuronic acid 2-O-sulfate presents low anticoagulant activity. Carbohydr. Polym. 2013, 94, 647–654. [Google Scholar] [CrossRef] [Green Version]
- Brito, A.S.; Cavalcante, R.S.; Palhares, L.C.G.F.; Hughes, A.J.; Andrade, G.P.V.; Yates, E.A.; Nader, H.B.; Lima, M.A.; Chavante, S.F. A non-hemorrhagic hybrid heparin/heparan sulfate with anticoagulant potential. Carbohydr. Polym. 2014, 99, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Yao, W.; Qiu, H.M.; Cheong, K.L.; Zhong, S. Advances in anti-cancer effects and underlying mechanisms of marine algae polysaccharides. Int. J. Biol. Macromol. 2022, 221, 472–485. [Google Scholar] [CrossRef]
- Liu, L. Improvement of Methods for Methylation Analysis of Acidic Polysaccharides. Master’s Thesis, Ocean University of China, Qingdao, China, 2014. [Google Scholar]
- Thomson, D.; Panagos, C.G.; Venkatasamy, R.; Moss, C.; Robinson, J.; Bavington, C.D.; Hogwood, J.; Mulloy, B.; Uhrin, D.; Spina, D.; et al. Structural characterization and anti-inflammatory activity of two novel polysaccharides from the sea squirt, Ascidiella aspersa. Pulm. Pharmacol. Ther. 2016, 40, 69–79. [Google Scholar] [CrossRef]
- Mauri, L.; Boccardi, G.; Torri, G.; Karfunkle, M.; Macchi, E.; Muzi, L.; Keire, D.; Guerrini, M. Qualification of HSQC methods for quantitative composition of heparin and low molecular weight heparins. J. Pharm. Biomed. Anal. 2017, 136, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, N.; Zuo, Z.; Li, S.; Zheng, W.; Shi, X.; Liu, Q.; Ma, T.; Yin, R.; Li, X.; et al. Five distinct fucan sulfates from sea cucumber Pattalus mollis: Purification, structural characterization and anticoagulant activities. Int. J. Biol. Macromol. 2021, 186, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Du, P.; Liu, X.; Cao, S.; Qin, L.; He, M.; He, X.; Mao, W. Anticoagulant Properties of a Green Algal Rhamnan-type Sulfated Polysaccharide and Its Low-molecular-weight Fragments Prepared by Mild Acid Degradation. Mar. Drugs 2018, 16, 445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; Wang, L.; Wang, L.; Yu, Q.; Ai, C.; Fu, Y.; Yan, C.; Wen, C.; Zhu, Z. Structural characterization and anticoagulant activity of two polysaccharides from Patinopecten yessoensis viscera. Int. J. Biol. Macromol. 2019, 136, 579–585. [Google Scholar] [CrossRef]
- Valcarcel, J.; Novoa-Carballal, R.; Perez-Martin, R.I.; Reis, R.L.; Vazquez, J.A. Glycosaminoglycans from marine sources as therapeutic agents. Biotechnol. Adv. 2017, 35, 711–725. [Google Scholar] [CrossRef]
- Brito, A.S.; Arimateia, D.S.; Souza, L.R.; Lima, M.A.; Santos, V.O.; Medeiros, V.P.; Ferreira, P.A.; Silva, R.A.; Ferreira, C.V.; Justo, G.Z.; et al. Anti-inflammatory properties of a heparin-like glycosaminoglycan with reduced anti-coagulant activity isolated from a marine shrimp. Bioorganic Med. Chem. 2008, 16, 9588–9595. [Google Scholar] [CrossRef]
- Cavalcante, R.S.; Brito, A.S.; Palhares, L.C.G.F.; Lima, M.A.; Cavalheiro, R.P.; Nader, H.B.; Sassaki, G.L.; Chavante, S.F. 2,3-Di-O-sulfo glucuronic acid: An unmodified and unusual residue in a highly sulfated chondroitin sulfate from Litopenaeus vannamei. Carbohydr. Polym. 2018, 183, 192–200. [Google Scholar] [CrossRef]










| ΔUA2S-GlcNS, 6S | ΔUA2S-GlcNAC, 6S | ΔUA-GlcNS, 6S | ΔUA-GlcNAC, 6S | ΔUA2S-GlcNS | ΔUA2S-GlcNAC | ΔUA-GlcNS | ΔUA-GlcNAC | IIA-IISglu | |
|---|---|---|---|---|---|---|---|---|---|
| Hep | 63.79% | 2.12% | 9.72% | 2.87% | 6.78% | 0.4% | 2.87% | 2.95% | 2.26% |
| F1 | 13.18% | - | 26.56% | 1.68% | 16.64% | 2.29% | 7.97% | 10.93% | 2.48% |
| F2 | 12.36% | - | 28.97% | 3.49% | 9.93% | 11.17% | 6.39% | 7.97% | 1.71% |
| RT | Methylated Sugar | Molar Ratio | Type of Linkage | Mass Fragments (m/z) |
|---|---|---|---|---|
| 16.458 | 2,3,5-Me3-Araf | 0.018 | Araf-(1→ | 43, 71, 87, 101, 117, 129, 145, 161 |
| 19.064 | 2,4-Me2-Araf | 0.035 | →3)-Araf-(1→ | 43, 85, 99, 101, 117, 127, 161, 159 |
| 22.147 | 2,3-Me2-Araf | 0.039 | →5)-Araf-(1→ | 43, 71, 87, 99, 101, 117, 129, 161, 189 |
| 22.667 | 2,3,4,6-Me4-Glcp | 0.208 | Glcp-(1→ | 43, 71, 87, 101, 117, 129, 145, 161, 205 |
| 24.057 | 2,3,4,6-Me4-Galp | 0.031 | Galp-(1→ | 43, 71, 87, 101, 117, 129, 145, 161, 205 |
| 29.813 | 2,3,6-Me3-Glcp | 0.431 | →4)-Glcp-(1→ | 43, 87, 99, 101, 113, 117, 129, 131, 161, 173, 233 |
| 30.342 | 2,4,6-Me3-Galp | 0.043 | →3)-Galp-(1→ | 43, 87, 99, 101, 117, 129, 161, 173, 233 |
| 33.712 | 2,6-Me2-Glcp | 0.054 | →3,4)-Glcp-(1→ | 43, 87, 97, 117, 159, 185 |
| 39.077 | 2,4-Me2-Galp | 0.141 | →3,6)-Galp-(1→ | 43, 87, 117, 129, 159, 189, 233 |
| RT | Methylated sugar | Molar ratio | Type of linkage | Mass fragments (m/z) |
| 32.968 | 2,3,4,6-Me4-Glcp | 0.698 | →4)-β-D-GlcpA-(1→ | 43, 87, 99, 101, 113, 117, 129, 131, 161, 173, 233 |
| 46.741 | 2,3,6-Me3-Glcp | 0.302 | →3)-β-D-GlcpNAc-(1→ | 43, 75, 100, 117, 129, 158, 171 |
| Residue | 1 | 2 | 3 | 4 | 5 | 6 | NAc |
|---|---|---|---|---|---|---|---|
| GlcA | 103.31/4.43 | 69.13/3.59 | 71.29/3.56 | 80.11/3.70 | 72.81 | 173.8 | - |
| GalNAc | 103.59/4.63 | 61.95/4.08 | 77.69/4.02 | 70.56/4.14 | 73.90/3.84 | 60.53/3.78 | 174.35; 22.35/2.04 |
| Residue | 1 | 2 | 3 | 4 | 5 | 6 | NAc |
| GlcA | 103.63/4.56 | 72.31/3.31 | 75.75/3.76 | 69.13/3.68 | 75.71/3.74 | - | - |
| IdoA | 99.52/5.21 | 77.70/4.34 | 72.15/3.70 | 76.74/4.14 | 74.73/4.93 | 174.54 | - |
| GlcN | 93.62/5.40 | 75.55/3.78 | 68.97/3.78 | 75.93/3.62 | 74.03/3.73 | 66.23/3.90 | 22.34/2.04 |
| Group | FXa/IU × mg−1 | FIIa/IU × mg−1 | Xa/IIa |
|---|---|---|---|
| F1 | 134.26 | 139.07 | 0.965 |
| F2 | 227.32 | 194.39 | 1.17 |
| Group | Begin Body Weight/g | Weight before Model/g |
|---|---|---|
| Control | 24.94 ± 1.46 | 33.70 ± 1.90 * |
| Model | 24.68 ± 1.01 | 35.42 ± 3.38 * |
| Heparin | 26.00 ± 1.74 | 34.48 ± 2.30 * |
| Clopidogrel | 23.76 ± 0.67 | 34.40 ± 1.33 * |
| F1 L | 24.66 ± 0.75 | 35.16 ± 2.08 * |
| F1 M | 24.34 ± 0.86 | 34.60 ± 1.32 * |
| F1 H | 24.88 ± 1.01 | 35.86 ± 0.81 * |
| F2 L | 24.86 ± 0.47 | 36.20 ± 1.49 * |
| F2 M | 25.22 ± 1.32 | 35.76 ± 1.40 * |
| F2 H | 24.38 ± 1.01 | 35.48 ± 2.22 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wang, Z.; Jia, X.; Li, R.; Chen, J.; Liu, X.; Song, B.; Zhong, S.; Qi, Y. Anticoagulant and Fibrinolytic Properties of Two Heparinoid Compounds Prepared from Shrimp Waste. Foods 2023, 12, 66. https://doi.org/10.3390/foods12010066
Chen J, Wang Z, Jia X, Li R, Chen J, Liu X, Song B, Zhong S, Qi Y. Anticoagulant and Fibrinolytic Properties of Two Heparinoid Compounds Prepared from Shrimp Waste. Foods. 2023; 12(1):66. https://doi.org/10.3390/foods12010066
Chicago/Turabian StyleChen, Jing, Zhuo Wang, Xuejing Jia, Rui Li, Jianping Chen, Xiaofei Liu, Bingbing Song, Saiyi Zhong, and Yi Qi. 2023. "Anticoagulant and Fibrinolytic Properties of Two Heparinoid Compounds Prepared from Shrimp Waste" Foods 12, no. 1: 66. https://doi.org/10.3390/foods12010066