A Risk–Benefit Analysis of First Nation’s Traditional Smoked Fish Processing
Abstract
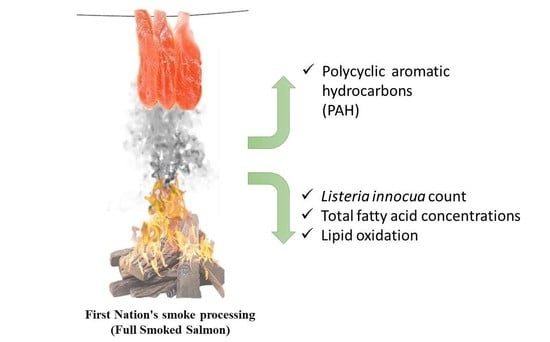
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Moisture Measurement
2.3. Sodium and Potassium Analysis
2.4. Polyaromatic Hydrocarbon (PAH) Analysis
2.5. Fatty Acid Analysis
2.6. Lipid Oxidation Analysis
2.7. L. innocua Inhibition Assay
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forsberg, N.D.; Stone, D.; Harding, A.; Harper, B.; Harris, S.; Matzke, M.M.; Cardenas, A.; Waters, K.M.; Anderson, K.A. Effect of native American fish smoking methods on dietary exposure to polycyclic aromatic hydrocarbons and possible risks to human health. J. Agri. Food Chem. 2012, 60, 6899–6906. [Google Scholar] [CrossRef] [Green Version]
- Kitts, D.D.; Chen, X.M.; Broda, P. Polyaromatic hydrocarbons of smoked cured muscle foods prepared by Canadian Tl’azt’en and Llheidli T’enneh First Nation communities. J. Toxicol. Environ. Health Part A 2012, 75, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Pirsaheb, M.; Irandost, M.; Asadi, F.; Fakhri, Y.; Asadi, A. Evaluation of polycyclic aromatic hydrocarbons (PAHs) in fish: A review and meta-analysis. Toxin Rev. 2020, 39, 205–213. [Google Scholar] [CrossRef]
- Wretling, S.; Eriksson, A.; Eskhult, G.A.; Larsson, B. Polycyclic aromatic hydrocarbons (PAHs) in Swedish smoked meat and fish. J. Food Compos. Anal. 2010, 23, 264–272. [Google Scholar] [CrossRef]
- Duedahl-Olesen, L.; Christensen, J.H.; Højgård, A.; Granby, K.; Timm-Heinrich, M. Influence of smoking parameters on the concentration of polycyclic aromatic hydrocarbons (PAHs) in Danish smoked fish. Food Addit. Contam. Part A 2010, 27, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Kafeelah, A.Y.; Lucy, N.E.; Kafayat, A.F.; Shehu, L.A.; Julius, I.A.; Titus, O.O. Influence of fish smoking methods on polycyclic aromatic hydrocarbons content and possible risks to human health. Afr. J. Food Sci. 2015, 9, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Farhadian, A.; Jinap, S.; Abas, F.; Sakar, Z.I. Determination of polycyclic aromatic hydrocarbons in grilled meat. Food Control 2010, 21, 606–610. [Google Scholar] [CrossRef]
- Ramesh, A.; Walker, S.A.; Hood, D.B.; Guillén, M.D.; Schneider, K.; Weyand, E.H. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int. J. Toxicol. 2004, 23, 301–333. [Google Scholar] [CrossRef]
- European Commission (EC). Commission Regulation (EU) No 835/2011 of 19 August 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. Off. J. Eur. Union 2011, 215, 4–8. [Google Scholar]
- Halek, F.; Nabi, G.; Kavousi, A. Polycyclic aromatic hydrocarbons study and toxic equivalency factor (TEFs) in Tehran, IRAN. Environ. Monit. Assess. 2008, 143, 303–311. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Zelinkova, Z.; Wenzl, T. The occurrence of 16 EPA PAHs in food—A review. Polycycl. Aromat. Compd. 2015, 35, 248–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essumang, D.K.; Dodoo, D.K.; Adjei, J.K. Effect of smoke generation sources and smoke curing duration on the levels of polycyclic aromatic hydrocarbon (PAH) in different suites of fish. Food Chem. Toxicol. 2013, 58, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Di Ciccio, P.; Meloni, D.; Festino, A.R.; Conter, M.; Zanardi, E.; Ghidini, S.; Vergara, A.; Mazzette, R.; Ianieri, A. Longitudinal study on the sources of Listeria monocytogenes contamination in cold-smoked salmon and its processing environment in Italy. Int. J. Food Microbiol. 2012, 158, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Rørvik, L.M. Listeria monocytogenes in the smoked salmon industry. Int. J. Food Microbiol. 2000, 62, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Hingston, P.; Johnson, K.; Kitts, D.; Wang, S. Safety and quality of fish and game meats prepared by First Nations communities in British Columbia, Canada. J. Food Prot. 2020, 83, 896–901. [Google Scholar] [CrossRef]
- Allen, K.J.; Chen, X.M.; Mesak, L.R.; Kitts, D.D. Antimicrobial activity of salmon extracts derived from traditional First Nations smoke processing. J. Food Prot. 2012, 75, 1878–1882. [Google Scholar] [CrossRef]
- Huynh, M.D.; Kitts, D.D. Evaluating nutritional quality of pacific fish species from fatty acid signatures. Food Chem. 2009, 114, 912–918. [Google Scholar] [CrossRef]
- Özogul, Y.; Özogul, F.h.; Çi˙çek, E.; Polat, A.; Kuley, E. Fat content and fatty acid compositions of 34 marine water fish species from the Mediterranean Sea. Int. J. Food Sci. Nutr. 2009, 60, 464–475. [Google Scholar] [CrossRef]
- Holub, D.J.; Holub, B.J. Omega-3 fatty acids from fish oils and cardiovascular disease. Mol. Cell. Biochem. 2004, 263, 217–225. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Geelen, A.; Brouwer, I.A.; Geleijnse, J.M.; Zock, P.L.; Katan, M.B. Effect of fish oil on heart rate in humans: A meta-analysis of randomized controlled trials. Circulation 2005, 112, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R.; Davis, J.M.; Steer, C.; Emmett, P.; Rogers, I.; Williams, C.; Golding, J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): An observational cohort study. Lancet 2007, 369, 578–585. [Google Scholar] [CrossRef]
- Jensen, C.L.; Voigt, R.G.; Llorente, A.M.; Peters, S.U.; Prager, T.C.; Zou, Y.L.; Rozelle, J.C.; Turcich, M.R.; Fraley, J.K.; Anderson, R.E.; et al. Effects of early maternal docosahexaenoic acid intake on neuropsychological status and visual acuity at five years of age of breast-fed term infants. J. Pediatr. 2010, 157, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Mukai, F.H.; Goldstein, B.D. Mutagenicity of malonaldehyde, a decomposition product of peroxidized polyunsaturated fatty acids. Science 1976, 191, 868–869. [Google Scholar] [CrossRef] [PubMed]
- Tenyang, N.; Tiencheu, B.; Womeni, H.M. Effect of smoking and refrigeration on lipid oxidation of Clupea harengus: A fish commonly consumed in Cameroon. Food Sci. Nutr. 2018, 6, 464–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianazza, E.; Brioschi, M.; Fernandez, A.M.; Banfi, C. Lipoxidation in cardiovascular diseases. Redox Biol. 2019, 23, 101119. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D. Dietary lipids and physiological function. In Bailey’s Industrial Oil and Fat Products; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 1–33. ISBN 9780471678496. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemist (AOAC). AOAC official method 976.21, fat (crude) in meat, rapid specific gravity method. In Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemist (AOAC): Washington, DC, USA, 1990. [Google Scholar]
- Delistraty, D. Toxic equivalency factor approach for risk assessment of polycyclic aromatic hydrocarbons. Toxicol. Environ. Chem. 1997, 64, 81–108. [Google Scholar] [CrossRef]
- Nisbet, I.C.; LaGoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Kitts, D.D.; Huynh, M.D.; Hu, C.; Trites, A.W. Season variation in nutrient composition of Alaskan walleye pollock. Can. J. Zool. 2004, 82, 1408–1415. [Google Scholar] [CrossRef]
- Lai, M.M.C.; Zhang, H.A.; Kitts, D.D. Ginseng prong added to broiler diets reduces lipid peroxidation in refrigerated and frozen stored poultry meats. Molecules 2021, 26, 4033. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.; Balfry, S.; Higgs, D.; Huang, C.-H.; Skura, B. Impact of iron-catalyzed dietary lipid peroxidation on growth performance, general health and flesh proximate and fatty acid composition of Atlantic salmon (Salmo salar L.) reared in seawater. Aquaculture 2006, 257, 534–557. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Cygnarowicz, M.L. A mathematical approach toward defining and calculating the duration of the lag phase. Food Microbiol. 1990, 7, 237–240. [Google Scholar] [CrossRef]
- Birkeland, S.; Skåra, T. Cold smoking of Atlantic salmon (Salmo salar) fillets with smoke condensate—An alternative processing technology for the production of smoked salmon. J. Food Sci. 2008, 73, S326–S332. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.; Nowak, B.; Behnke, A.; Seidel, A.; Lampen, A. Effect-based and chemical analysis of polycyclic aromatic hydrocarbons in smoked meat: A practical food-monitoring approach. Food Addit. Contam. Part A 2009, 26, 1104–1112. [Google Scholar] [CrossRef]
- Flick, G.J., Jr.; Kuhn, D.D. Smoked, cured, and dried fish. In The Seafood Industry; Granata, L.A., Flick, G.J., Jr., Martin, R.E., Eds.; Springer: Boston, MA, USA, 2012; pp. 404–426. [Google Scholar] [CrossRef]
- Goulas, A.E.; Kontominas, M.G. Effect of salting and smoking-method on the keeping quality of chub mackerel (Scomber japonicus): Biochemical and sensory attributes. Food Chem. 2005, 93, 511–520. [Google Scholar] [CrossRef]
- Visciano, P.; Perugini, M.; Amorena, M.; IanieriI, A. Polycyclic aromatic hydrocarbons in fresh and cold-smoked Atlantic salmon fillets. J. Food Prot. 2006, 69, 1134–1138. [Google Scholar] [CrossRef]
- Basak, S.; ŞEngÖR, G.F.; KarakoÇ, F.T. The detection of potential carcinogenic PAH using HPLC procedure in two different smoked fish, case study: Istanbul/Turkey. Turk. J. Fish. Aquat. Sci. 2010, 10, 351–355. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6853, Fluorene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Fluorene (accessed on 2 November 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 995, Phenanthrene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Phenanthrene (accessed on 2 November 2022).
- US Environmental Protection Agency (EPA). Napthalene. Available online: https://www.epa.gov/sites/defalult/files/2016-09/documents/naphthalene.pdf (accessed on 2 November 2022).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 9171, Chrysene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Chrysene (accessed on 2 November 2022).
- Masuda, Y.; Kuratsune, M. Polycyclic aromatic hydrocarbons in smoked fish, “katsuobushi”. Jpn. J. Cancer Res. 1971, 62, 27–30. [Google Scholar] [CrossRef]
- Sei, K.; Wang, Q.; Tokumura, M.; Suzuki, S.; Miyake, Y.; Amagai, T. Polycyclic aromatic hydrocarbons and their halogenated derivatives in a traditional smoke-dried fish product in Japan: Occurrence and countermeasures. ACS Food Sci. Technol. 2021, 1, 960–966. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Polycyclic aromatic hydrocarbons in food—Scientific opinion of the panel on contaminants in the food chain. EFSA J. 2008, 6, 724. [Google Scholar] [CrossRef]
- Codex Alimentariius Commission (CAC). Code of practice for the reduction of contamination of food with polycyclic aromatic hydrocarbons (PHA) from smoking and direct drying processes (CAC/RCP68-2009). In Prevention and Reduction of Food and Feed Contamination, 1st ed.; Joint FAO/WHO Food Standards Program: Rome, Italy, 2009. [Google Scholar]
- Espe, M.; Nortvedt, R.; Lie, Ø.; Hafsteinsson, H. Atlantic salmon (Salmo salar, L) as raw material for the smoking industry. II: Effect of different smoking methods on losses of nutrients and on the oxidation of lipids. Food Chem. 2002, 77, 41–46. [Google Scholar] [CrossRef]
- Blanchet, C.; Lucas, M.; Julien, P.; Morin, R.; Gingras, S.; Dewailly, E. Fatty acid composition of wild and farmed Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss). Lipids 2005, 40, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef]
- Kjaer, M.A.; Todorcević, M.; Torstensen, B.E.; Vegusdal, A.; Ruyter, B. Dietary n-3 HUFA affects mitochondrial fatty acid beta-oxidation capacity and susceptibility to oxidative stress in Atlantic salmon. Lipids 2008, 43, 813–827. [Google Scholar] [CrossRef]
- Guichardant, M.; Chantegrel, B.; Deshayes, C.; Doutheau, A.; Moliere, P.; Lagarde, M. Specific markers of lipid peroxidation issued from n−3 and n−6 fatty acids. Biochem. Soc. Trans. 2004, 32, 139–140. [Google Scholar] [CrossRef]
- Bilgin, Ş.; Ünlüsayin, M.; İzci, L.; Günlü, A. The determination of the shelf life and some nutritional components of gilthead seabream (Sparus aurata L., 1758) after cold and hot smoking. Turk. J. Vet. Anim. Sci. 2008, 32, 9. [Google Scholar]
- Arvanitoyannis, I.S.; Kotsanopoulos, K.V. Smoking of fish and seafood: History, methods and effects on physical, nutritional and microbiological properties. Food Bioprocess Technol. 2012, 5, 831–853. [Google Scholar] [CrossRef]
- Smailagić, A.; Ristivojević, P.; Dimkić, I.; Pavlović, T.; Dabić Zagorac, D.; Veljović, S.; Fotirić Akšić, M.; Meland, M.; Natić, M. Radical scavenging and antimicrobial properties of polyphenol rich waste wood extracts. Foods 2020, 9, 319. [Google Scholar] [CrossRef] [Green Version]
- Suñen, E. Minimum inhibitory concentration of smoke wood extracts against spoilage and pathogenic micro-organisms associated with foods. Lett. Appl. Microbiol. 1998, 27, 45–48. [Google Scholar] [CrossRef]


| PAH | Non-Smoked 2 | FN Half-Smoked | FN Fully Smoked |
|---|---|---|---|
| Naphthalene | ND 3 | 229 ± 76 a | 351 ± 87 b |
| Acenaphthylene | ND | 351 ± 92 a | 586 ± 210 b |
| Acenaphthene | ND | 31 ± 15 a | 117 ± 18 b |
| Fluorene | ND | 122 ± 46 a | 532 ± 85 b |
| Phenanthrene | ND | 382 ± 137 a | 3191 ± 373 b |
| Anthracene | ND | 76 ± 15 a | 538 ± 65 b |
| Fluoranthene | ND | 46 ± 15 a | 308 ± 46 b |
| Pyrene | ND | 31 ± 15 a | 273 ± 37 b |
| Benzo[α]anthracene | ND | ND | 13 ± 4 b |
| Chrysene | ND | ND | 27 ± 12 b |
| Benzo[b]fluoranthene | ND | ND | ND |
| Benzo[k]fluoranthene | ND | ND | ND |
| Benzo[α]pyrene | ND | ND | ND |
| Total PAH | ND | 1145 ± 382 a | 5972 ± 544 b |
| Total BAP eqi. 4 | ND | 0.0109 | 3.97 |
| Fatty Acid | Non-Smoked | Half-Smoked | Fully Smoked | Commercial |
|---|---|---|---|---|
| SFA | 3.56 ± 0.33 a | 2.33 ± 0.29 b | 2.58 ± 1.08 b | 1.89 ± 0.67 |
| PUFA | 5.18 ± 0.41 a | 2.88 ± 0.31 b | 2.89 ± 0.33 b | 0.06 ± 0.03 |
| cis-MUFA | 8.19 ± 0.80 a | 6.47 ± 0.77 b | 6.74 ± 4.29 b | 2.54 ± 0.40 |
| C18:2 n-6 | 0.45 ± 0.09 a | 0.21 ± 0.02 b | 0.24 ± 0.18 b | 0.11 ± 0.00 |
| C20:4 n-6 | 0.21 ± 0.06 a | 0.05 ± 0.00 b | 0.06 ± 0.04 b | 0.06 ± 0.02 |
| C20:5 n-3 | 1.20 ± 0.09 a | 0.72 ± 0.07 b | 0.78 ± 0.60 b | 0.73 ± 0.09 |
| C22:6 n-3 | 2.20 ± 0.17 a | 1.30 ± 0.12 b | 1.20 ± 0.70 b | 1.70 ± 0.25 |
| Omega-3 | 4.50 ± 0.36 a | 2.50 ± 0.23 b | 2.60 ± 0.80 b | 2.80 ± 0.34 |
| Omega-6 | 0.77 ± 0.17 a | 0.33 ± 0.04 b | 0.38 ± 0.28 b | 0.28 ± 0.03 |
| Omega-3/6 | 5.80 | 7.58 | 6.86 | 9.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitts, D.D.; Pratap-Singh, A.; Singh, A.; Chen, X.; Wang, S. A Risk–Benefit Analysis of First Nation’s Traditional Smoked Fish Processing. Foods 2023, 12, 111. https://doi.org/10.3390/foods12010111
Kitts DD, Pratap-Singh A, Singh A, Chen X, Wang S. A Risk–Benefit Analysis of First Nation’s Traditional Smoked Fish Processing. Foods. 2023; 12(1):111. https://doi.org/10.3390/foods12010111
Chicago/Turabian StyleKitts, David D., Anubhav Pratap-Singh, Anika Singh, Xiumin Chen, and Siyun Wang. 2023. "A Risk–Benefit Analysis of First Nation’s Traditional Smoked Fish Processing" Foods 12, no. 1: 111. https://doi.org/10.3390/foods12010111