Effects of Drying Methods and Temperatures on the Quality of Chestnut Flours
Abstract
:1. Introduction
2. Materials and Methods
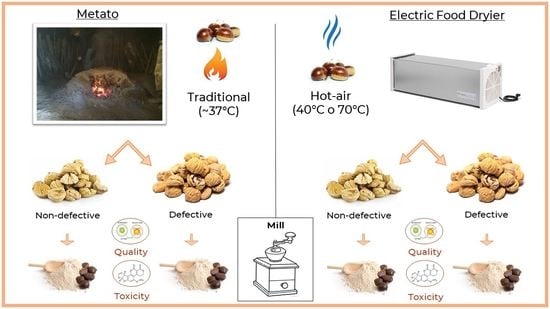
2.1. Collection of Chestnut Flour
- By the traditional “metato” drier, working with a wood fire, covered by ashes, where the fluctuating temperature recorded by a data logger localized into the fruit container ranged from a minimum of 30 °C to a maximum of 70 °C, with a mean value of 37 °C;
- By a laboratory oven (Biosec, Tauro, Camisano Vicentino, Italy) at a constant temperature of 70 °C;
- By a commercial air dessicator (Biosec, Tauro, Camisano Vicentino, Italy) at a steady temperature of 40 °C.
- Non-defective: any color alteration was present neither outside nor inside, with the chestnut appearing totally white;
- Defective: alterations present in 25 ± 2% of the volume of the nuts with the flesh turned brown/chalky white.
2.2. Moisture Determination
2.3. Sample Extraction for Colorimetric Assays
2.4. Determination of Antioxidant Power
2.5. Determination Phenolic Content
2.6. Analysis of Sugars
2.7. Aflatoxin Content Determination
2.8. Toxic Volatile Compounds by SPME-GC-MS
2.9. Statistics
3. Results and Discussion
3.1. Moisture Determination
3.2. Determination of Antioxidant Power
3.3. Determination Phenolic Content
3.4. Analyses of Sugars
3.5. Aflatoxin Determination
3.6. Toxic Volatile Compounds by SPME-GC-MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Correia, P.; Leitão, A.; Beirão-da-Costa, M.L. The effect of drying temperatures on morphological and chemical properties of dried chestnuts flours. J. Food Eng. 2009, 90, 325–332. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 12 January 2021).
- Pietri, A.; Rastelli, S.; Mulazzi, A.; Bertuzzi, T. Aflatoxins and ochratoxin A in dried chestnuts and chestnut flour produced in Italy. Food Control 2012, 25, 601–606. [Google Scholar] [CrossRef]
- Demirkesen, I.; Mert, B.; Sumnu, G.; Sahin, S. Utilization of chestnut flour in gluten-free bread formulations. J. Food Eng. 2010, 101, 329–336. [Google Scholar] [CrossRef]
- Paciulli, M.; Rinaldi, M.; Cirlini, M.; Scazzina, F.; Chiavaro, E. Chestnut flour addition in commercial gluten-free bread: A shelf-life study. LWT—Food Sci. Technol. 2016, 70, 88–95. [Google Scholar] [CrossRef]
- Mir, S.A.; Bosco, S.J.D.; Shah, M.A. Technological and nutritional properties of gluten-free snacks based on brown rice and chestnut flour. J. Saudi Soc. Agric. Sci. 2019, 18, 89–94. [Google Scholar] [CrossRef]
- Littardi, P.; Paciulli, M.; Carini, E.; Rinaldi, M.; Rodolfi, M.; Chiavaro, E. Quality evaluation of chestnut flour addition on fresh pasta. LWT 2020, 126, 109303. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Shi, G.; Yang, H.; Wang, X.; Zhao, H.; Zhao, S. Effects of drying methods on the nutritional aspects, flavor, and processing properties of Chinese chestnuts. J. Food Sci. Technol. 2018, 55, 3391–3398. [Google Scholar] [CrossRef]
- Cirlini, M.; Dall’Asta, C.; Silvanini, A.; Begh, D.; Fabbri, A.; Galaverna, G.; Ganino, T. Volatile fingerprinting of chestnut flours from traditional Emilia Romagna (Italy) cultivars. Food Chem. 2012, 134, 662–668. [Google Scholar] [CrossRef]
- Prencipe, S.; Siciliano, I.; Gatti, C.; Gullino, M.L.; Garibaldi, A.; Spadaro, D. Chestnut drying is critical in determining aspergillus flavus growth and aflatoxin contamination. Toxins 2018, 10, 530. [Google Scholar] [CrossRef] [Green Version]
- De Vasconcelos, M.C.B.M.; Bennett, R.N.; Rosa, E.A.S.; Ferreira-Cardoso, J.V. Composition of European chestnut (Castanea sativa Mill.) and association with health effects: Fresh and processed products. J. Sci. Food Agric. 2010, 90, 1578–1589. [Google Scholar] [CrossRef]
- Massantini, R.; Moscetti, R.; Frangipane, M.T. Evaluating progress of chestnut quality: A review of recent developments. Trends Food Sci. Technol. 2021, 113, 245–254. [Google Scholar] [CrossRef]
- Li, Q.; Shi, X.; Zhao, Q.; Cui, Y.; Ouyang, J.; Xu, F. Effect of cooking methods on nutritional quality and volatile compounds of Chinese chestnut (Castanea mollissima Blume). Food Chem. 2016, 201, 80–86. [Google Scholar] [CrossRef]
- Zhang, X.; Levia, D.F.; Ebikade, E.O.; Chang, J.; Vlachos, D.G.; Wu, C. The impact of differential lignin S/G ratios on mutagenicity and chicken embryonic toxicity. J. Appl. Toxicol. 2021, 42, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Henríquez, C.; Almonacid, S.; Chiffelle, I.; Valenzuela, T.; Araya, M.; Cabezas, L.; Simpson, R.; Speisky, H. Determinación de la capacidad antioxidante, contenido de fenoles totales y composición mineral de diferentes tejidos de frutos de cinco variedades de manzana cultivadas en Chile. Chil. J. Agric. Res. 2010, 70, 523–536. [Google Scholar] [CrossRef]
- Ferracini, C.; Ferrari, E.; Pontini, M.; Saladini, M.A.; Alma, A. Effectiveness of Torymus sinensis: A successful long-term control of the Asian chestnut gall wasp in Italy. J. Pest Sci. 2018, 92, 353–359. [Google Scholar] [CrossRef]
- Lione, G.; Danti, R.; Fernandez-Conradi, P.; Ferreira-Cardoso, J.V.; Lefort, F.; Marques, G.; Meyer, J.B.; Prospero, S.; Radócz, L.; Robin, C.; et al. The emerging pathogen of chestnut Gnomoniopsis castaneae: The challenge posed by a versatile fungus. Eur. J. Plant Pathol. 2019, 153, 671–685. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Paolacci, A.; Vannini, A. Endophytism of Sclerotinia pseudotuberosa: PCR assay for specific detection in chestnut tissues. Mycol. Res. 2005, 109, 96–102. [Google Scholar] [CrossRef]
- Overy, D.P.; Seifert, K.A.; Savard, M.E.; Frisvad, J.C. Spoilage fungi and their mycotoxins in commercially marketed chestnuts. Int. J. Food Microbiol. 2003, 88, 69–77. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Rastelli, S.; Pietri, A. Aspergillus and Penicillium toxins in chestnuts and derived products produced in Italy. Food Control 2015, 50, 876–880. [Google Scholar] [CrossRef]
- Rodrigues, P.; Venâncio, A.; Lima, N. Incidence and diversity of the fungal genera Aspergillus and Penicillium in Portuguese almonds and chestnuts. Eur. J. Plant Pathol. 2013, 137, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Edite Bezerra da Rocha, M.; da Freire, F.C.O.; Erlan Feitosa Maia, F.; Izabel Florindo Guedes, M.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Shephard, G.S. Impact of mycotoxins on human health in developing countries. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 146–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Rodriguez, C.; Bastianelli, G.; Caccia, R.; Bedini, G.; Massantini, R.; Moscetti, R.; Thomidis, T.; Vannini, A. Impact of ‘brown rot’ caused by Gnomoniopsis castanea on chestnut fruits during the post-harvest process: Critical phases and proposed solutions. J. Sci. Food Agric. 2022, 102, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Gazzetta Ufficiale della Repubblica Italiana. Available online: https://www.gazzettaufficiale.it/eli/gu/1985/06/21/145/sg/pdf (accessed on 7 April 2022).
- Alothman, M.; Bhat, R.; Karim, A.A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Pugliese, C.; Sirtori, F.; Calamai, L.; Franci, O. The evolution of volatile compounds profile of “Toscano” dry-cured ham during ripening as revealed by SPME-GC-MS approach. J. Mass Spectrom. 2010, 45, 1056–1064. [Google Scholar] [CrossRef]
- Disciplinare di Produzione “Farina di Neccio della Garfagnana”. Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaArticolo?art.progressivo=0&art.idArticolo=1&art.versione=1&art.codiceRedazionale=001A1546&art.dataPubblicazioneGazzetta=2001-02-20&art.idGruppo=0&art.idSottoArticolo1=10&art.idSottoArticolo=1&art.flagTipoArticolo=1 (accessed on 7 April 2022).
- Disciplinare di Produzione “Farina di Castagne della Lunigiana”. Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaArticolo?art.progressivo=0&art.idArticolo=1&art.versione=1&art.codiceRedazionale=06A02338&art.dataPubblicazioneGazzetta=2006-03-10&art.idGruppo=0&art.idSottoArticolo1=10&art.idSottoArticolo=1&art.flagTipoArticolo=1 (accessed on 7 April 2022).
- Gazzetta Ufficiale della Repubblica Italiana “Disciplina per la Lavorazione e Commercio dei Cereali, Degli Sfarinati, del Pane e delle Paste Alimentari”. Available online: https://www.gazzettaufficiale.it/eli/id/1967/07/29/067U0580/sg (accessed on 7 April 2022).
- Blokhina, O.; Virolainen, E.; Fagerstedt, K. V Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Wanyo, P.; Huaisan, K.; Chamsai, T. Phenolic Compounds and Antioxidant Properties of Thai rice Paddy Herb as Affected by Different Drying Temperature. Int. J. Agric. Technol. 2018, 14, 423–440. [Google Scholar]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M. Reactive oxygen species (ROS): Beneficial companions of plants’ developmental processes. Front. Plant Sci. 2016, 7, 1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, K.; Adhikari, S.; Tarafdar, A.; Kumar, R.; Saha, S.; Ghosh, P. Reactive Oxygen Species and Antioxidant Defence Systems in Plants: Role and Crosstalk Under Biotic Stress BT—Sustainable Agriculture in the Era of Climate Change. In Sustainable Agriculture in the Era of Climate Change; Roychowdhury, R., Choudhury, S., Hasanuzzaman, M., Srivastava, S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 265–292. ISBN 978-3-030-45669-6. [Google Scholar]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Piga, A.; Del Caro, A.; Corda, G. From Plums to Prunes: Influence of Drying Parameters on Polyphenols and Antioxidant Activity. J. Agric. Food Chem. 2003, 51, 3675–3681. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Míguez Bernárdez, M.M.; De la Montaña Miguélez, J.; García Queijeiro, J. HPLC determination of sugars in varieties of chestnut fruits from Galicia (Spain). J. Food Compos. Anal. 2004, 17, 63–67. [Google Scholar] [CrossRef]
- Lo Piccolo, E.; Landi, M.; Ceccanti, C.; Mininni, A.N.; Marchetti, L.; Massai, R.; Guidi, L.; Remorini, D. Nutritional and nutraceutical properties of raw and traditionally obtained flour from chestnut fruit grown in Tuscany. Eur. Food Res. Technol. 2020, 246, 1867–1876. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars-metabolism, sensing and abiotic stress a complex network in the life of plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [Green Version]
- Piccini, C.; Cai, G.; Dias, M.C.; Romi, M.; Longo, R.; Cantini, C. UV-B radiation affects photosynthesis-related processes of two Italian olea Europaea (L.) varieties differently. Plants 2020, 9, 1712. [Google Scholar] [CrossRef]
- Conde, C.; Silva, P.; Agasse, A.; Lemoine, R.; Delrot, S.; Tavares, R.; Geros, H. Utilization and Transport of Mannitol in Olea europaea and Implications for Salt Stress Tolerance. Plant Cell Physiol. 2006, 48, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Ennajeh, M.; Vadel, A.M.; Khemira, H. Osmoregulation and osmoprotection in the leaf cells of two olive cultivars subjected to severe water deficit. Acta Physiol. Plant. 2009, 31, 711–721. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, Y.; Li, X.; Zhao, J.; Guo, N.; Xing, H. Metabolomics analysis of soybean hypocotyls in response to phytophthora sojae infection. Front. Plant Sci. 2018, 871, 1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saiz-Fernández, I.; Milenković, I.; Berka, M.; Černý, M.; Tomšovský, M.; Brzobohatý, B.; Kerchev, P. Integrated proteomic and metabolomic profiling of Phytophthora cinnamomi attack on sweet chestnut (Castanea sativa) reveals distinct molecular reprogramming proximal to the infection site and away from it. Int. J. Mol. Sci. 2020, 21, 8525. [Google Scholar] [CrossRef] [PubMed]
- Dinis, L.T.; Peixoto, F.; Zhang, C.; Martins, L.; Costa, R.; Gomes-Laranjo, J. Physiological and biochemical changes in resistant and sensitive chestnut (Castanea) plantlets after inoculation with Phytophthora cinnamomi. Physiol. Mol. Plant Pathol. 2011, 75, 146–156. [Google Scholar] [CrossRef]
- Valente, S.; Meloni, G.R.; Prencipe, S.; Spigolon, N.; Somenzi, M.; Fontana, M.; Gullino, M.L.; Spadaro, D. Effect of Drying Temperatures and Exposure Times on Aspergillus flavus Growth and Aflatoxin Production on Artificially Inoculated Hazelnuts. J. Food Prot. 2020, 83, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Krist, S.; Unterweger, H.; Bandion, F.; Buchbauer, G. Volatile compound analysis of SPME headspace and extract samples from roasted Italian chestnuts (Castanea sativa Mill.) using GC-MS. Eur. Food Res. Technol. 2004, 219, 470–473. [Google Scholar] [CrossRef]
- Hoydonckx, H.E.; Van Rhijn, W.M.; Van Rhijn, W.; De Vos, D.E.; Jacobs, P.A. Furfural and Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 285–309. [Google Scholar] [CrossRef]
- Fiege, H. Cresols and Xylenols. In Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000; pp. 673–710. [Google Scholar] [CrossRef]
- Inamdar, A.A.; Moore, J.C.; Cohen, R.I.; Bennett, J.W. A Model to Evaluate the Cytotoxicity of the Fungal Volatile Organic Compound 1-octen-3-ol in Human Embryonic Stem Cells. Mycopathologia 2012, 173, 13–20. [Google Scholar] [CrossRef] [PubMed]






| 1-Octen-3-ol | Furfural | Guaiacol | O-Cresol | |||||
|---|---|---|---|---|---|---|---|---|
| RT | Area | RT | Area | RT | Area | RT | Area | |
| Traditional non-defective | 22.003 | 1,298,711 | 22.926 | 3,288,748* | 32.584 | 8,686,673* | 35.439 | 836,714 |
| Traditional defective | 22.001 | 640,837 | 22.922 | 53,749 | 32.582 | 4,408,542 | 35.435 | 2,602,744* |
| Hot air 70 °C non-defective | 21.999 | 431,202 | 22.922 | 438,989 | 32.584 | 1,097,308 | 35.437 | 334,842 |
| Hot air 40 °C defective | 22.003 | 5,320,343* | 22.924 | 585,345 | 32.586 | 678,755 | 35.435 | 177,180 |
| Hot air 70 °C non-defective | 22.001 | 259,093 | 22.926 | 251,179 | 32.586 | 2,334,425 | 35.435 | 728,489 |
| Hot air 40 °C defective | 22.001 | 609,126 | 22.928 | 1,590,987 | 32.584 | 3,752,913 | 35.435 | 1,416,660 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti, V.; Salusti, P.; Romi, M.; Cantini, C. Effects of Drying Methods and Temperatures on the Quality of Chestnut Flours. Foods 2022, 11, 1364. https://doi.org/10.3390/foods11091364
Conti V, Salusti P, Romi M, Cantini C. Effects of Drying Methods and Temperatures on the Quality of Chestnut Flours. Foods. 2022; 11(9):1364. https://doi.org/10.3390/foods11091364
Chicago/Turabian StyleConti, Veronica, Patrizia Salusti, Marco Romi, and Claudio Cantini. 2022. "Effects of Drying Methods and Temperatures on the Quality of Chestnut Flours" Foods 11, no. 9: 1364. https://doi.org/10.3390/foods11091364