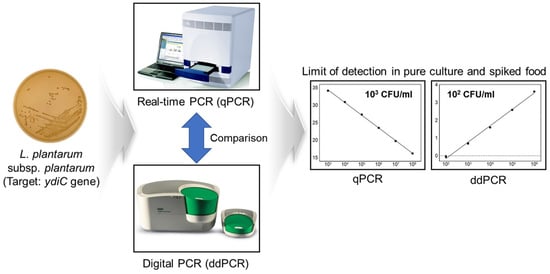
Comparison of Real-Time PCR and Droplet Digital PCR for the Quantitative Detection of Lactiplantibacillus plantarum subsp. plantarum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Primer and Probe Design
2.3. qPCR Assay
2.4. ddPCR Assay
2.5. Artificially Contaminated Milk Sample
3. Results and Discussion
3.1. Specificity of Primer by In Silico PCR
3.2. Evaluation of the Specificity and Sensitivity by qPCR
3.3. Evaluation of Specificity and Sensitivity by ddPCR
3.4. Comparison of Sensitivity and Linearity of qPCR and ddPCR Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furet, J.P.; Quénée, P.; Tailliez, P. Molecular quantification of lactic acid bacteria in fermented milk products using real-time quantitative PCR. Int. J. Food Microbiol. 2004, 97, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Guarino, A.; Guandalini, S.; Vecchio, A.L. Probiotics for prevention and treatment of diarrhea. J. Clin. Gastroenterol. 2015, 49, S37–S45. [Google Scholar] [CrossRef] [PubMed]
- Peña, J.A.; Rogers, A.B.; Ge, Z.; Ng, V.; Li, S.Y.; Fox, J.G.; Versalovic, J. Probiotic Lactobacillus spp. diminish Helicobacter hepaticus-induced inflammatory bowel disease in interleukin-10-deficient mice. Infect. Immun. 2005, 73, 912–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.; Chang, H.C.; Kim, H.Y. Complete genome sequence of Lactobacillus plantarum EM, a putative probiotic strain with the cholesterol-lowering effect and antimicrobial activity. Curr. Microbiol. 2020, 77, 1871–1882. [Google Scholar] [CrossRef]
- Martino, M.E.; Bayjanov, J.R.; Caffrey, B.E.; Wels, M.; Joncour, P.; Hughes, S.; Gillet, B.; Kleerebezem, M.; van Hijum, S.A.F.T.; Leulier, F. Nomadic lifestyle of Lactobacillus plantarum revealed by comparative genomics of 54 strains isolated from different habitats. Environ. Microbiol. 2016, 18, 4974–4989. [Google Scholar] [CrossRef]
- Jeong, C.H.; Sohn, H.; Hwang, H.; Lee, H.J.; Kim, T.W.; Kim, D.S.; Kim, C.S.; Han, S.G.; Hong, S.W. Comparison of the probiotic potential between Lactiplantibacillus plantarum isolated from kimchi and standard probiotic strains isolated from different sources. Foods 2021, 10, 2125. [Google Scholar] [CrossRef]
- Fernandes, P.; Loureiro, D.; Monteiro, V.; Ramos, C.; Nero, L.A.; Todorov, S.D.; Guerreiro, J.S. Lactobacillus plantarum isolated from cheese: Production and partial characterization of bacteriocin B391. Ann. Microbiol. 2017, 67, 433–442. [Google Scholar] [CrossRef]
- Wang, W.; Ma, H.; Yu, H.; Qin, G.; Tan, Z.; Wang, Y.; Pang, H. Screening of Lactobacillus plantarum subsp. plantarum with potential probiotic activities for inhibiting ETEC K88 in weaned piglets. Molecules 2020, 25, 4481. [Google Scholar] [CrossRef]
- Giri, S.S.; Kim, H.J.; Kim, S.G.; Kim, S.W.; Kwon, J.; Lee, S.B.; Woo, K.J.; Jung, W.J.; Kim, M.J.; Sukumaran, V.; et al. Effects of dietary Lactiplantibacillus plantarum subsp. plantarum L7, alone or in combination with Limosilactobacillus reuteri P16, on growth, mucosal immune responses, and disease resistance of Cyprinus carpio. Probiotics Antimicrob. Proteins 2021, 13, 1747–1758. [Google Scholar] [CrossRef]
- Jin, Y.J.; Park, Y.K.; Cho, M.S.; Lee, E.S.; Park, D.S. New insight and metrics to understand the ontogeny and succession of Lactobacillus plantarum subsp. plantarum and Lactobacillus plantarum subsp. argentoratensis. Sci. Rep. 2018, 8, 6029. [Google Scholar] [CrossRef] [Green Version]
- Bringel, F.; Castioni, A.; Olukoya, D.K.; Felis, G.E.; Torriani, S.; Dellaglio, F. Lactobacillus plantarum subsp. argentoratensis subsp. nov., isolated from vegetable matrices. Int. J. Syst. Evol. Microbiol. 2005, 55, 1629–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.; Kim, H.B.; Yang, S.M.; Kim, D.; Kim, H.Y. Real-time PCR assay for detecting Lactobacillus plantarum group using species/subspecies-specific genes identified by comparative genomics. LWT 2021, 138, 110789. [Google Scholar] [CrossRef]
- Huang, C.H.; Chen, C.C.; Lin, Y.C.; Chen, C.H.; Lee, A.Y.; Liou, J.S.; Gu, C.T.; Huang, L. The mutL gene as a genome-wide taxonomic marker for high resolution discrimination of Lactiplantibacillus plantarum and its closely related taxa. Microorganisms 2021, 9, 1570. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Daliri, E.B.M.; Chelliah, R.; Park, B.J.; Lim, J.S.; Baek, M.A.; Nam, Y.S.; Seo, K.H.; Jin, Y.G.; Oh, D.H. Development of a multiplex real-time PCR for simultaneous detection of Bacillus cereus, Listeria monocytogenes, and Staphylococcus aureus in food samples. J. Food Saf. 2019, 39, e12558. [Google Scholar] [CrossRef] [Green Version]
- Porcellato, D.; Narvhus, J.; Skeie, S.B. Detection and quantification of Bacillus cereus group in milk by droplet digital PCR. J. Microbiol. Methods 2016, 127, 1–6. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.; Wang, T.; Dong, Q.; Li, J.; Niu, C. Detection of 12 common food-borne bacterial pathogens by taq man real-time PCR using a single set of reaction conditions. Front. Microbiol. 2019, 10, 222. [Google Scholar] [CrossRef]
- Pierboni, E.; Curcio, L.; Tovo, G.R.; Torricelli, M.; Rondini, C. Evaluation of systems for nopaline synthase terminator in fast and standard real-time PCR to screen genetically modified organisms. Food Anal. Methods 2016, 9, 1009–1019. [Google Scholar] [CrossRef]
- Fraiture, M.A.; Gobbo, A.; Marchesi, U.; Verginelli, D.; Papazova, N.; Roosens, N.H.C. Development of a real-time PCR marker targeting a new unauthorized genetically modified microorganism producing protease identified by DNA walking. Int. J. Food Microbiol. 2021, 354, 109330. [Google Scholar] [CrossRef]
- Lefever, S.; Rihani, A.; Van der Meulen, J.; Pattyn, F.; Van Maerken, T.; Van Dorpe, J.; Hellemans, J.; Vandesompele, J. Cost-effective and robust genotyping using double-mismatch allele-specific quantitative PCR. Sci. Rep. 2019, 9, 2150. [Google Scholar] [CrossRef]
- Torricelli, M.; Sebastiani, C.; Ciullo, M.; Ceccobelli, S.; Chiappini, B.; Vaccari, G.; Capocefalo, A.; Conte, M.; Giovannini, S.; Lasagna, E.; et al. PRNP polymorphisms in eight local goat populations/breeds from central and southern Italy. Animals 2021, 11, 333. [Google Scholar] [CrossRef]
- Hindson, B.J.; Ness, K.D.; Masquelier, D.A.; Belgrader, P.; Heredia, N.J.; Makarewicz, A.J.; Bright, I.J.; Lucero, M.Y.; Hiddessen, A.L.; Legler, T.C.; et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem. 2011, 83, 8604–8610. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xia, Q.; Yin, Y.; Wang, Z. Comparison of droplet digital PCR and quantitative PCR assays for quantitative detection of Xanthomonas citri subsp. citri. PLoS ONE 2016, 11, e0159004. [Google Scholar] [CrossRef] [Green Version]
- Ricchi, M.; Bertasio, C.; Boniotti, M.B.; Vicari, N.; Russo, S.; Tilola, M.; Bellotti, M.A.; Bertasi, B. Comparison among the quantification of bacterial pathogens by qPCR, dPCR, and cultural methods. Front. Microbiol. 2017, 8, 1174. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhai, S.; Gao, H.; Xiao, F.; Li, Y.; Wu, G.; Wu, Y. Development and assessment of a duplex droplet digital PCR method for quantification of GM rice Kemingdao. Anal. Bioanal. Chem. 2021, 413, 4341–4351. [Google Scholar] [CrossRef]
- Persson, S.; Eriksson, R.; Lowther, J.; Ellström, P.; Simonsson, M. Comparison between RT droplet digital PCR and RT real-time PCR for quantification of noroviruses in oysters. Int. J. Food Microbiol. 2018, 284, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Rački, N.; Dreo, T.; Gutierrez-Aguirre, I.; Blejec, A.; Ravnikar, M. Reverse transcriptase droplet digital PCR shows high resilience to PCR inhibitors from plant, soil and water samples. Plant Methods 2014, 10, 42. [Google Scholar] [CrossRef]
- Xu, W.; Shen, P.; Li, R.; Liu, B.; Yang, L. Development of an event-specific droplet digital PCR assay for quantification and evaluation of the transgene DNAs in trace samples of GM PRNP-knockout goat. Foods 2022, 11, 868. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Rana, A.; Sung, W.; Munir, M. Competitiveness of quantitative polymerase chain reaction (qPCR) and droplet digital polymerase chain reaction (ddPCR) technologies, with a particular focus on detection of antibiotic resistance genes (ARGs). Appl. Microbiol. 2021, 1, 426–444. [Google Scholar] [CrossRef]
- Rice, L.M.; Robb, L.L.; Hartman, D.A.; Anderson, J.R.; Kading, R.C. Application of the droplet digital polymerase chain reaction (ddPCR) platform for detection and quantification of vertebrate host DNA in engorged mosquitoes. J. Med. Entomol. 2019, 56, 1150–1153. [Google Scholar] [CrossRef]
- Kim, E.; Kim, D.; Yang, S.-M.; Kim, H.-Y. Validation of probiotic species or subspecies identity in commercial probiotic products using high-resolution PCR method based on large-scale genomic analysis. Food Res. Int. 2022, 154, 111011. [Google Scholar] [CrossRef]
- In silico PCR amplification. Available online: http://insilico.ehu.es/PCR/ (accessed on 8 March 2022).
- Gómez-Rojo, E.M.; Romero-Santacreu, L.; Jaime, I.; Rovira, J. A novel real-time PCR assay for the specific identification and quantification of Weissella viridescens in blood sausages. Int. J. Food Microbiol. 2015, 215, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Dupas, E.; Legendre, B.; Olivier, V.; Poliakoff, F.; Manceau, C.; Cunty, A. Comparison of real-time PCR and droplet digital PCR for the detection of Xylella fastidiosa in plants. J. Microbiol. Methods 2019, 162, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Gu, X.; Zhong, Q.; Duan, L.; Zhou, A. Absolute quantification of Vibrio parahaemolyticus by multiplex droplet digital PCR for simultaneous detection of tlh, tdh and ureR based on single intact cell. Food Control 2020, 114, 107207. [Google Scholar] [CrossRef]
- Cristiano, D.; Peruzy, M.F.; Aponte, M.; Mancusi, A.; Proroga, Y.T.R.; Capuano, F.; Murru, N. Comparison of droplet digital PCR vs real-time PCR for Yersinia enterocolitica detection in vegetables. Int. J. Food Microbiol. 2021, 354, 109321. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wu, C. Molecular discrimination of Lactobacillus plantarum group using comparative sequence analysis of the dnaJ gene and as a target for developing novel species-specific PCR primers. J. Chin. Soc. Anim. Sci. 2016, 45, 45–55. [Google Scholar]
- Galanis, A.; Kourkoutas, Y.; Tassou, C.C.; Chorianopoulos, N. Detection and identification of probiotic Lactobacillus plantarum strains by multiplex PCR using RAPD-derived primers. Int. J. Mol. Sci. 2015, 16, 25141–25153. [Google Scholar] [CrossRef] [PubMed]
- Schwendimann, L.; Kauf, P.; Fieseler, L.; Gantenbein-Demarchi, C.; Miescher Schwenninger, S. Development of a quantitative PCR assay for rapid detection of Lactobacillus plantarum and Lactobacillus fermentum in cocoa bean fermentation. J. Microbiol. Methods 2015, 115, 94–99. [Google Scholar] [CrossRef]
- Broeders, S.; Huber, I.; Grohmann, L.; Berben, G.; Taverniers, I.; Mazzara, M.; Roosens, N.; Morisset, D. Guidelines for validation of qualitative real-time PCR methods. Trends Food Sci. Technol. 2014, 37, 115–126. [Google Scholar] [CrossRef]
- Gobert, G.; Cotillard, A.; Fourmestraux, C.; Pruvost, L.; Miguet, J.; Boyer, M. Droplet digital PCR improves absolute quantification of viable lactic acid bacteria in faecal samples. J. Microbiol. Methods 2018, 148, 64–73. [Google Scholar] [CrossRef]
- Hansen, S.J.Z.; Morovic, W.; DeMeules, M.; Stahl, B.; Sindelar, C.W. Absolute enumeration of probiotic strains Lactobacillus acidophilus NCFM® and Bifidobacterium animalis subsp. lactis Bl-04® via chip-based digital PCR. Front. Microbiol. 2018, 9, 704. [Google Scholar] [CrossRef] [Green Version]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Kado, Y.; Takada, T.; Matsumoto, K.; Tanaka, R. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl. Environ. Microbiol. 2004, 70, 167–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, H.; Dong, K.; Rao, L.; Zhao, L.; Wu, X.; Wang, Y.; Liao, X. Quantitative detection of viable but nonculturable state Escherichia coli O157:H7 by ddPCR combined with propidium monoazide. Food Control 2020, 112, 107140. [Google Scholar] [CrossRef]
- Wang, M.; Yang, J.; Gai, Z.; Huo, S.; Zhu, J.; Li, J.; Wang, R.; Xing, S.; Shi, G.; Shi, F.; et al. Comparison between digital PCR and real-time PCR in detection of Salmonella Typhimurium in milk. Int. J. Food Microbiol. 2018, 266, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, N.; Lv, J.; Zhu, P.; Pan, X.; Hu, J.; Wu, W.; Li, S.; Li, H. Comparing the performance of conventional PCR, RTQ-PCR, and droplet digital PCR assays in detection of Shigella. Mol. Cell. Probes 2020, 51, 101531. [Google Scholar] [CrossRef] [PubMed]
- Hougs, L.; Gatto, F.; Goerlich, O.; Grohmann, L.; Lieske, K.; Mazzara, M.; Narendja, F.; Ovesna, J.; Papazova, N.; Scholtens, I.M.J.; et al. Verification of analytical methods for GMO testing when implementing interlaboratory validated methods. In Testing and Analysis of GMO-Containing Foods and Feed; CRC Press: Boca Raton, FL, USA, 2017; pp. 245–266. [Google Scholar]
- Pinheiro, L.B.; Coleman, V.A.; Hindson, C.M.; Herrmann, J.; Hindson, B.J.; Bhat, S.; Emslie, K.R. Evaluation of a droplet digital polymerase chain reaction format for DNA copy number quantification. Anal. Chem. 2012, 84, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Villamil, C.; Calderon, M.N.; Arias, M.M.; Leguizamon, J.E. Validation of droplet digital polymerase chain reaction for Salmonella spp. quantification. Front. Microbiol. 2020, 11, 1512. [Google Scholar] [CrossRef]
- Mairiang, D.; Songjaeng, A.; Hansuealueang, P.; Malila, Y.; Lertsethtakarn, P.; Silapong, S.; Poolpanichupatam, Y.; Klungthong, C.; Chin-Inmanu, K.; Thiemmeca, S.; et al. Application of one-step reverse transcription droplet digital PCR for dengue virus detection and quantification in clinical specimens. Diagnostics 2021, 11, 639. [Google Scholar] [CrossRef]
- Luo, J.; Li, J.; Yang, H.; Yu, J.; Wei, H. Accurate detection of methicillin-resistant Staphylococcus aureus in mixtures by use of single-bacterium duplex droplet digital PCR. J. Clin. Microbiol. 2017, 55, 2946–2955. [Google Scholar] [CrossRef] [Green Version]



| Species | Strain No. |
|---|---|
| Lactiplantibacillus plantarum subsp. plantarum | KACC 11451 |
| Lactiplantibacillus plantarum subsp. argentoratensis | KACC 12404 |
| Lactiplantibacillus paraplantarum | KACC 12373 |
| Lactiplantibacillus pentosus | KACC 12428 |
| Apilactobacillus kunkeei | KACC 19371 |
| Bifidobacterium animalis subsp. animalis | KCTC 3125 |
| Bifidobacterium animalis subsp. lactis | KCTC 5854 |
| Bifidobacterium bifidum | KCTC 3418 |
| Bifidobacterium bifidum | KCTC 3440 |
| Bifidobacterium breve | KACC 16639 |
| Bifidobacterium breve | KCTC 3419 |
| Bifidobacterium longum subsp. infantis | KCTC 3249 |
| Bifidobacterium longum subsp. longum | KCCM 11953 |
| Companilactobacillus crustorum | KACC 16344 |
| Companilactobacillus farciminis | KACC 12423 |
| Companilactobacillus heilongjiangensis | KACC 18741 |
| Enterococcus avium | NCCP 10761 |
| Enterococcus avium | KACC 10788 |
| Enterococcus casseliflavus | KCTC 3638 |
| Enterococcus cecorum | KACC 13884 |
| Enterococcus durans | KCTC 13289 |
| Enterococcus durans | KACC 10787 |
| Enterococcus faecalis | KCTC 3206 |
| Enterococcus faecalis | KACC 11859 |
| Enterococcus faecium | KCTC 13225 |
| Enterococcus faecium | KACC 11954 |
| Enterococcus faecium | KACC 10782 |
| Enterococcus gilvus | KACC 13847 |
| Enterococcus malodoratus | KACC 13883 |
| Enterococcus mundtii | KCTC 3630 |
| Enterococcus mundtii | KACC 13824 |
| Enterococcus saccharolyticus | KACC 10783 |
| Enterococcus thailandicus | KCTC 13134 |
| Fructilactobacillus lindneri | KACC 12445 |
| Lacticaseibacillus brantae | KACC 17260 |
| Lacticaseibacillus camelliae | KACC 17261 |
| Lacticaseibacillus casei | KACC12413 |
| Lacticaseibacillus casei | KCTC 13086 |
| Lacticaseibacillus casei | KCTC 3110 |
| Lacticaseibacillus chiayiensis | NBRC 112906 |
| Lacticaseibacillus manihotivorans | KACC 12380 |
| Lacticaseibacillus pantheris | KACC 12395 |
| Lacticaseibacillus paracasei subsp. paracasei | KCTC 3165 |
| Lacticaseibacillus paracasei subsp. tolerans | KCTC 3074 |
| Lacticaseibacillus rhamnosus | KACC 11953 |
| Lacticaseibacillus rhamnosus | KCTC 13088 |
| Lacticaseibacillus sharpeae | KACC 11462 |
| Lactobacillus acetotolerans | KACC 12447 |
| Lactobacillus acidophilus | KACC 12419 |
| Lactobacillus acidophilus | KCTC 3164 |
| Lactobacillus amylolyticus | KACC 12374 |
| Lactobacillus amylophilus | KACC 11430 |
| Lactobacillus amylovorus | KACC 12435 |
| Lactobacillus brevis | KCTC 3498 |
| Lactobacillus curvatus subsp. curvatus | KACC 12415 |
| Lactobacillus delbrueckii subsp. bulgaricus | KACC 12420 |
| Lactobacillus delbrueckii subsp. delbrueckii | KACC 13439 |
| Lactobacillus delbrueckii subsp. lactis | KACC 12417 |
| Lactobacillus gallinarum | KACC 12370 |
| Lactobacillus gasseri | KCTC 3163 |
| Lactobacillus gasseri | KACC 12424 |
| Lactobacillus helveticus | KACC 12418 |
| Lactobacillus jensenii | KCTC 5194 |
| Lactobacillus johnsonii | KCTC 3801 |
| Lactococcus lactis subsp. lactis | KCTC 3769 |
| Lactococcus lactis subsp. lactis | KCTC 2013 |
| Latilactobacillus sakei subsp. sakei | KCTC 3603 |
| Lentilactobacillus buchneri | KACC 12416 |
| Lentilactobacillus parabuchneri | KACC 12363 |
| Leuconostoc carnosum | KCTC 3525 |
| Leuconostoc citreum | KCTC 3526 |
| Leuconostoc fallax | KACC 12303 |
| Leuconostoc gelidum | KACC 12256 |
| Leuconostoc gelidum subsp. aenigmaticum | MGB 1000TE |
| Leuconostoc gelidum subsp. gasicomitatum | KACC 13854 |
| Leuconostoc gelidum subsp. gelidum | KCTC 3527 |
| Leuconostoc holzapfelii | KACC 17729 |
| Leuconostoc lactis | KCTC 3528 |
| Leuconostoc mesenteroides subsp. dextranicum | KACC 12315 |
| Leuconostoc mesenteroides subsp. mesenteroides | KCTC 3505 |
| Leuconostoc pseudomesenteroides | KACC 12304 |
| Levilactobacillus zymae | KACC 16349 |
| Ligilactobacillus acidipiscis | KACC 12394 |
| Ligilactobacillus agilis | KACC 12433 |
| Ligilactobacillus ruminis | KACC 12429 |
| Ligilactobacillus salivarius | KCTC 3600 |
| Limosilactobacillus fermentum | KACC 11441 |
| Limosilactobacillus fermentum | KCTC 3112 |
| Limosilactobacillus fermentum | KCTC 5049 |
| Limosilactobacillus mucosae | KACC 12381 |
| Limosilactobacillus reuteri | KCTC 3594 |
| Loigolactobacillus coryniformis subsp. coryniformis | KACC 12411 |
| Streptococcus salivarius subsp. thermophilus | KACC 11857 |
| Weissella beninensis | KACC 18586 |
| Weissella cibaria | KCTC 3746 |
| Weissella confusa | KACC 11841 |
| Weissella halotolerans | KACC 11843 |
| Weissella hellenica | KACC 11842 |
| Weissella kandleri | KACC 11844 |
| Weissella koreensis | KACC 11853 |
| Weissella minor | KCTC 3604 |
| Weissella paramesenteroides | KACC 11847 |
| Weissella soli | KACC 11848 |
| Weissella thailandensis | KACC 11849 |
| Weissella viridescens | KACC 11850 |
| Name | Sequence (5′-3′) | Size (bp) |
|---|---|---|
| Plantarum_F | GGT GGC TGG TTG AGT GAT CT | 150 bp |
| Plantarum_R | GCC GAT ACC GTT GGA AAT TA | |
| Plantarum_P | FAM-ACA GCT TGT TCT ACT AAC CGG CCT AGT CC-MGB |
| Conc. (CFU/mL) | Pure Culture 1 | Spiked Food Sample | ||
|---|---|---|---|---|
| qPCR (Ct) | ddPCR (Copies) | qPCR (Ct) | ddPCR (Copies) | |
| 108 | 16.37 ± 0.04 (9) | Saturated 2 | 16.13 ± 0.08 (9) | Saturated |
| 107 | 19.83 ± 0.03 (9) | Saturated | 19.52 ± 0.05 (9) | Saturated |
| 106 | 23.37 ± 0.02 (9) | 3287.78 ± 106.98 (9) | 23.46 ± 0.07 (9) | 4310 ± 295 (9) |
| 105 | 27.08 ± 0.15 (9) | 318.44 ± 10.49 (9) | 27.44 ± 0.12 (9) | 427.22 ± 60.31 (9) |
| 104 | 30.67 ± 0.08 (9) | 31.64 ± 1.9 (9) | 31.25 ± 0.16 (9) | 40.46 ± 6.58 (9) |
| 103 | 34.11 ± 0.31 (9) | 3.42 ± 0.62 (9) | 34.79 ± 0.3 (9) | 3.98 ± 0.59 (9) |
| 102 | ND 3 | 0.4 ± 0.11 (9) | ND | 0.25 ± 0.14 (9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, C.-H.; Kim, E.; Yang, S.-M.; Kim, D.-S.; Suh, S.-M.; Lee, G.-Y.; Kim, H.-Y. Comparison of Real-Time PCR and Droplet Digital PCR for the Quantitative Detection of Lactiplantibacillus plantarum subsp. plantarum. Foods 2022, 11, 1331. https://doi.org/10.3390/foods11091331
Choi C-H, Kim E, Yang S-M, Kim D-S, Suh S-M, Lee G-Y, Kim H-Y. Comparison of Real-Time PCR and Droplet Digital PCR for the Quantitative Detection of Lactiplantibacillus plantarum subsp. plantarum. Foods. 2022; 11(9):1331. https://doi.org/10.3390/foods11091331
Chicago/Turabian StyleChoi, Chang-Hun, Eiseul Kim, Seung-Min Yang, Da-Som Kim, Seung-Man Suh, Ga-Young Lee, and Hae-Yeong Kim. 2022. "Comparison of Real-Time PCR and Droplet Digital PCR for the Quantitative Detection of Lactiplantibacillus plantarum subsp. plantarum" Foods 11, no. 9: 1331. https://doi.org/10.3390/foods11091331