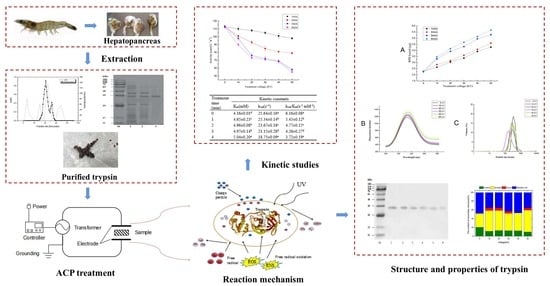
Changes in Biochemical Properties and Activity of Trypsin-like Protease (Litopenaeus vannamei) Treated by Atmospheric Cold Plasma (ACP)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trypsin Extraction from Shrimp
2.1.1. Preparation of Hepatopancreas Extract
2.1.2. Ammonium Sulfate Fraction Preparation
2.1.3. Purification of Trypsin
2.2. Evaluation of Trypsin Activity
2.3. Determination of Protein Concentration
2.4. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.5. Sample Preparation and Atmospheric Cold Plasma Treatment
2.6. Kinetic Studies
2.7. The Effect of ACP on Temperature and pH Profile of Trypsin
2.8. Assay Surface Hydrophobicity
2.9. Assay the Total Content of Sulfhydryl
2.10. Determination of Circular Dichroism
2.11. Intrinsic Fluorescence Spectroscopy
2.12. Determination of Particle Size
2.13. Statistical Analysis
3. Results and Discussion
3.1. Purification of Trypsin from Shrimps (Litopenaeus vannamei)
3.2. Purity and Molecular Weight of Trypsin from Shrimps (Litopenaeus vannamei)
3.3. Effect of ACP on the Activity of Trypsin
3.4. Kinetic Study
3.5. Effect of ACP on Temperature and pH of Trypsin
3.6. Effect of ACP on the Surface Hydrophobicity of Trypsin
3.7. Effect of ACP on the Total Sulfhydryl of Trypsin
3.8. Effect of ACP on the Fluorescence Intensity of Trypsin
3.9. Effect of ACP on the Particle Size Distribution of Trypsin
3.10. The Effect of ACP on the SDS-PAGE of Trypsin
3.11. Effect of ACP on the Secondary Structure of Trypsin
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, A.; Benjakul, S. Proteolysis and its control using protease inhibitors in fish and fish products: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Singh, B. Inhibition of post-mortem fish muscle softening and degradation using legume seed proteinase inhibitors. J. Food Sci. Technol. 2020, 57, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Weiss, F.U.; Halangk, W.; Lerch, M.M. New advances in pancreatic cell physiology and pathophysiology. Best Pract. Res. Clin. Gastroenterol. 2008, 22, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Brauer, J.; Salazar-Leyva, J.; Bringas, L.; Rouzaud-Sández, O. Effect of dietary protein on muscle collagen, collagenase and shear force of farmed white shrimp (Litopenaeus vannamei). Eur. Food Res. Technol. 2003, 217, 277–280. [Google Scholar] [CrossRef]
- Sriket, C.; Benjakul, S.; Visessanguan, W. Characterisation of proteolytic enzymes from muscle and hepatopancreas of fresh water prawn (Macrobrachium rosenbergii). J. Sci. Food Agric. 2011, 91, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Senphan, T.; Benjakul, S. Compositions and yield of lipids extracted from hepatopancreas of Pacific white shrimp (Litopenaeus vannamei) as affected by prior autolysis. Food Chem. 2012, 13, 829–835. [Google Scholar] [CrossRef]
- Cao, W.; Tan, C.; Zhan, X.; Li, H.; Zhang, C. Ultraviolet irradiation and gradient temperature assisted autolysis for protein recovery from shrimp head waste. Food Chem. 2014, 164, 136–141. [Google Scholar] [CrossRef]
- Xiao, H.; Lin, Q.; Li, Y.; Zhao, M. Advances on applied studies of protease inhibitor in gene engineering. Biotechnol. Bull. 2004, 6, 22–25. [Google Scholar]
- Bijina, B.; Chellappan, S.; Krishna, J.G.; Basheer, S.M.; Elyas, K.K.; Bahkali, A.H.; Chandrasekaran, M. Protease inhibitor from Moringa oleifera with potential for use as therapeutic drug and as seafood preservative. Saudi J. Biol. Sci. 2011, 18, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Klomklao, S.; Benjakul, S. Effect of trypsin inhibitor in adzuki bean (Vigna angularis) on proteolysis and gel properties of threadfin bream (Nemipterus bleekeri). LWT Food Sci. Technol. 2015, 63, 906–911. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2012, 353, 2885–2904. [Google Scholar] [CrossRef]
- Miao, W.H.; Nyaisaba, B.M.; Koddy, J.K.; Chen, M.L.; Hatab, S.; Deng, S.G. Effect of cold atmospheric plasma on the physicochemical and functional properties of myofibrillar protein from Alaska pollock (Theragra chalcogramma). Int. J. Food Sci. Technol. 2020, 55, 517–525. [Google Scholar] [CrossRef]
- Misra, N.N.; Jo, C. Applications of cold plasma technology for microbiological safety in meat industry. Trends Food Sci. Technol. 2017, 64, 74–86. [Google Scholar] [CrossRef]
- Bußler, S.; Ehlbeck, J.; Schlüter, O. Pre-drying treatment of plant related tissues using plasma processed air: Impact on enzyme activity and quality attributes of cut apple and potato. Innov. Food Sci. Emerg. 2016, 40, 78–86. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Misra, N.N.; Cullen, P.J. Kinetics of tomato peroxidase inactivation by atmospheric pressure cold plasma based on dielectric barrier discharge. Innov. Food Sci. Emerg. 2013, 19, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Nyaisaba, B.M.; Miao, W.H.; Hatab, S.; Siloam, A.; Chen, M.L.; Deng, S.G. Effects of cold atmospheric plasma on squid proteases and gel properties of protein concentrate from squid (Argentinus ilex) mantle. Food Chem. 2019, 291, 68–76. [Google Scholar] [CrossRef]
- Khantaphant, S.; Benjakul, S. Purification and characterization of trypsin from the pyloric caeca of brownstripe red snapper (Lutjanus vitta). Food Chem. 2010, 120, 658–664. [Google Scholar] [CrossRef]
- Kristjansson, M.M. Purification and characterization of trypsin from the pyloric caeca of rainbow trout (Oncorhynchus mykiss). J. Agric. Food Chem. 1991, 39, 1738–1742. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the folin reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Yoshida, A.; Bae, I.; Sonoda, H.; Masuo, R.; Oda, S.; Cao, M.J.; Osatomi, K.; Hara, K. Characterization of gelatinolytic enzymes in the skeletal muscle of red sea bream pagrus major. Fish. Sci. 2009, 75, 1317–1322. [Google Scholar] [CrossRef]
- Cao, Y.G.; Zhao, J.; Xiong, Y.L.L. Coomassie brilliant blue-binding: A simple and effective method for the determination of water-insoluble protein surface hydrophobicity. Anal. Methods 2016, 8, 790–795. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Xu, X.M. Antioxidant and cryoprotective effects of Amur sturgeon skin gelatin hydrolysate in unwashed fish mince. Food Chem. 2015, 181, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Farasat, M.; Arjmand, S.; Siadat, S.O.R.; Sefidbakht, Y.; Ghomi, H. The effect of non-thermal atmospheric plasma on the production and activity of recombinant phytase enzyme. Sci. Rep. 2018, 8, 16647. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, F.; Ji, W.; Yokoyama, W.; Shoemaker, C.F.; Zhu, S.; Xia, W.S. Functional properties of maillard reaction products of rice protein hydrolysates with mono-, oligo- and polysaccharides. Food Hydrocolloid 2013, 30, 53–60. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Tatsumi, E.; Isobe, S. High-pressure treatment effects on proteins in soy milk. LWT—Food Sci. Technol. 2004, 38, 7–14. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Zhao, L.; Qiu, Y.; Zhuang, Y. Influence of processing conditions on reducing γ-aminobutyric acid content during fortified milk production. Food Res. Int. 2015, 72, 215–222. [Google Scholar] [CrossRef]
- Jones, R.G.; Landon, J. Enhanced pepsin digestion: A novel process for purifying antibody F(ab’)(2) fragments in high yield from serum. J. Immunol. Methods 2002, 263, 57–74. [Google Scholar] [CrossRef]
- Senphan, T.; Benjakul, S.; Kishimura, H. Purification and characterization of trypsin from hepatopancreas of Pacific white shrimp. J. Food Biochem. 2015, 39, 388–397. [Google Scholar] [CrossRef]
- Poonsin, T.; Simpson, B.K.; Benjakul, S.; Visessanguan, W.; Yoshida, A.; Osatomi, K.; Klomklao, S. Anionic trypsin from the spleen of albacore tuna (Thunnus alalunga): Purification, biochemical properties and its application for proteolytic degradation of fish muscle. Int. J. Biol. Macromol. 2019, 133, 971–979. [Google Scholar] [CrossRef]
- Honjo, I.; Kimura, S.; Nonaka, M. Purification and characterization of trypsin-like enzyme from shrimp Penaeus indicus. Nippon Suisan Gakk 1990, 5, 1627–1634. [Google Scholar] [CrossRef] [Green Version]
- Perera, E.; Rodríguez-Viera, L.; Perdomo-Morales, R.; Montero-Alejo, V.; Moyano, F.J.; Martínez-Rodríguez, G.; Mancera, J.M. Trypsin isozymes in the lobster Panulirus argus (Latreille, 1804): From molecules to physiology. J. Comp. Physiol. B 2015, 185, 17–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.Z.; Cheng, Z.Z.; Zhang, J.H.; Nasiru, M.M.; Wang, Y.B.; Fu, L.L. Atmospheric cold plasma treatment of soybean protein isolate: Insights into the structural, physicochemical, and allergenic characteristics. J. Food Sci. 2021, 86, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Attri, P.; Lee, I.; Oh, J.; Yun, J.H.; Park, J.; Choi, E.; Lee, W. Structural and functional analysis of lysozyme after treatment with dielectric barrier discharge plasma and atmospheric pressure plasma jet. Sci. Rep. 2017, 7, 1027. [Google Scholar] [CrossRef] [PubMed]
- Li, H.P.; Wang, L.Y.; Li, G.; Jin, L.; Le, P.S.; Zhao, H.X.; Xing, X.H.; Bao, C.Y. Manipulation of lipase activity by the helium radio-frequency, atmospheric-pressure glow discharge plasma jet. Plasma Processes Polym. 2011, 8, 224–229. [Google Scholar] [CrossRef]
- Ali, A.; Ashraf, Z.; Kumar, N.; Rafiq, M.; Jabeen, F.; Park, J.; Choi, K.; Lee, S.; Seo, S.-Y.; Choi, E.; et al. Influence of plasma-activated compounds on melanogenesis and tyrosinase activity. Sci. Rep. 2016, 6, 21779. [Google Scholar] [CrossRef]
- Puač, N.; Škoro, N.; Spasic, K.; Živković, S.; Milutinovic, M.; Malovic, G.; Petrović, Z. Activity of catalase enzyme in Paulownia tomentosa seeds during the process of germination after treatments with low pressure plasma and plasma activated water. Plasma Processes Polym. 2017, 15, 1700082. [Google Scholar] [CrossRef]
- Segat, A.; Misra, N.N.; Cullen, P.J.; Innocente, N. Effect of atmospheric pressure cold plasma (ACP) on activity and structure of alkaline phosphatase. Food Bioprod. Process. 2016, 98, 181–188. [Google Scholar] [CrossRef]
- Ji, Y.B.; Wang, F.L. Optimization of trypsin extraction technology of Allium cepa L. polysaccharide by response surface methodology and the antitumor effects through immunomodulation. Bioengineered 2021, 12, 382–391. [Google Scholar] [CrossRef]
- Meinlschmidt, P.; Ueberham, E.; Lehmann, J.; Reineke, K.; Schluter, O.; Schweiggert-Weisz, U.; Eisner, P. The effects of pulsed ultraviolet light, cold atmospheric pressure plasma, and gamma-irradiation on the immunoreactivity of soy protein isolate. Innov. Food Sci. Emerg. 2016, 38, 374–383. [Google Scholar] [CrossRef]
- Benjakul, S.; Morrissey, M.T. Protein hydrolysates for Pacific whiting solid wastes. J. Agric. Food Chem. 1997, 45, 3423–3430. [Google Scholar] [CrossRef]
- Liu, Z.W.; Manzoor, M.F.; Tan, Y.C.; Inam-ur-Raheem, M.; Aadil, R.M. Effect of dielectric barrier discharge (DBD) plasma on the structure and antioxidant activity of bovine serum albumin (BSA). Int. J. Food Sci. Technol. 2020, 55, 2824–2831. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, B.; Guo, J.; Nie, W.; Zhou, H.; Li, P.J.; Zhou, K.; Xu, B.C. Effects of low voltage electrostatic field on the microstructural damage and protein structural changes in prepared beef steak during the freezing process. Meat Sci. 2021, 179, 108527. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.K.; Lee, S.; Bin Song, K. Effect of high-pressure treatment on the molecular properties of mushroom polyphenoloxidase. LWT-Food Sci. Technol. 2002, 35, 315–318. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Wu, J.H.; Hu, X.S.; Zhi, X.; Liao, X.J. Alterations in the activity and structure of pectin methylesterase treated by high pressure carbon dioxide. J. Agric. Food Chem. 2009, 57, 1890–1895. [Google Scholar] [CrossRef] [PubMed]
- Attri, P.; Choi, E. Influence of reactive oxygen species on the enzyme stability and activity in the presence of ionic liquids. PLoS ONE 2013, 8, e75096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, C.; Bovay, C.; Rouvet, M.; Shojaei-Rami, S.; Kolodziejczyk, E. Whey protein soluble aggregates from heating with NaCl: Physicochemical, interfacial, and foaming properties. Langmuir 2007, 23, 4155–4166. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, N.K.; Raghavarao, K.; Balasubramaniam, V.M.; Niranjan, K.; Knorr, D. Opportunities and challenges in high pressure processing of foods. Crit. Rev. Food Sci. 2007, 47, 69–112. [Google Scholar] [CrossRef] [PubMed]
- Ekezie, F.C.; Sun, D.W.; Cheng, J.H. Altering the IgE binding capacity of king prawn (Litopenaeus Vannamei) tropomyosin through conformational changes induced by cold argon-plasma jet. Food Chem. 2019, 300, 125143. [Google Scholar] [CrossRef]
- Fang, M.X.; Luo, X.Y.; Xiong, S.B.; Yin, T.; Hu, Y.; Liu, R.; Du, H.Y.; Liu, Y.M.; You, J. In vitro trypsin digestion and identification of possible cross-linking sites induced by transglutaminase (TGase) of silver carp (Hypophthalmichthys molitrix) surimi gels with different degrees of cross-linking. Food Chem. 2021, 364, 130433. [Google Scholar] [CrossRef]
- Setsuhara, Y.; Cho, K.; Shiratani, M.; Sekine, M.; Hori, M. Plasma interactions with aminoacid (l-alanine) as a basis of fundamental processes in plasma medicine. Curr. Appl. Phys. 2013, 13, 59–63. [Google Scholar] [CrossRef]
- Priya Arjunan, K.; Morss Clyne, A. Hydroxyl radical and hydrogen peroxide are primarily responsible for dielectric barrier discharge plasma-induced angiogenesis. Plasma Processes Polym. 2011, 8, 1154–1164. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Shen, J.; Li, X.; Ding, L.; Ma, J.; Lan, Y.; Xia, W.; Cheng, C.; Sun, Q.; et al. Effects and mechanism of atmospheric-pressure dielectric barrier discharge cold plasmaon Lactate Dehydrogenase (LDH) Enzyme. Sci. Rep. 2015, 5, 10031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi, H.; Wako, H.; Yura, K.; Go, M.; Mimuro, M. Significance of a two-domain structure in subunits of phycobiliproteins revealed by the normal mode analysis. Biophys. J. 2000, 79, 1587–1600. [Google Scholar] [CrossRef] [Green Version]
- Surowsky, B.; Fischer, A.; Schlueter, O.; Knorr, D. Cold plasma effects on enzyme activity in a model food system. Innov. Food Sci. Emerg. 2013, 19, 146–152. [Google Scholar] [CrossRef]
- Huang, M.; Li, X.; Tong, P.; Gao, J.; Yuan, J.; Yang, A.; Chen, H.; Wu, Y. Potential allergenicity assessment after bovine apo-α-actalbumin binding to calcium ion. J. Food Biochem. 2020, 44, e13340. [Google Scholar] [CrossRef]
- Attri, P.; Kumar, N.; Park, J.H.; Yadav, D.K.; Choi, S.; Uhm, H.S.; Kim, I.T.; Choi, E.H.; Lee, W. Influence of reactive species on the modification of biomolecules generated from the soft plasma. Sci. Rep. 2015, 5, 8221. [Google Scholar] [CrossRef] [Green Version]







| Purification Step | Total Activity (units) | Total Protein (mg) | Specific Activity (Units/mg Protein) | Purity (Fold) | Yield (%) |
|---|---|---|---|---|---|
| Crude enzyme | 1876.3 | 5922.4 | 0.3 | 1.0 | 100 |
| Ammonium sulfate | 1544.6 | 512.1 | 3.0 | 10.0 | 82.3 |
| Q-Sepharose Fast Flow | 468.05 | 18.5 | 25.3 | 84.3 | 24.9 |
| Treatment Time (min) | Kinetic Constants | ||
|---|---|---|---|
| Km (mM) | kcat (s−1) | kcat/Km (s−1 mM−1) | |
| 0 | 4.16 ± 0.01 d | 25.64 ± 0.16 a | 6.16 ± 0.08 a |
| 1 | 4.65 ± 0.23 c | 25.34 ± 0.14 b | 5.45 ± 0.12 b |
| 2 | 4.96 ± 0.08 b | 23.67 ± 0.18 c | 4.77 ± 0.15 c |
| 3 | 4.97 ± 0.14 b | 21.15 ± 0.28 d | 4.26 ± 0.27 d |
| 4 | 5.04 ± 0.20 a | 18.75 ± 0.09 e | 3.72 ± 0.19 e |
| Treatment Time (min) | Treatment Voltage (kV) | ||||
|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | |
| 0 | 8.563 ± 0.006 b | 8.563 ± 0.006 b | 8.563 ± 0.006 b | 8.567 ± 0.021 b | 8.563 ± 0.006 b |
| 1 | 8.517 ± 0.015 b | 8.533 ± 0.006 b | 8.527 ± 0.006 b | 8.517 ± 0.006 b | 8.523 ± 0.006 b |
| 2 | 8.523 ± 0.021 ab | 8.527 ± 0.006 b | 8.533 ± 0.021 b | 8.503 ± 0.015 b | 8.513 ± 0.021 b |
| 3 | 8.527 ± 0.015 ab | 8.527 ± 0.006 b | 8.533 ± 0.021 b | 8.503 ± 0.015 b | 8.503 ± 0.015 b |
| 4 | 8.563 ± 0.032 a | 8.540 ± 0.026 ab | 8.543 ± 0.006 ab | 8.527 ± 0.006 ab | 8.523 ± 0.015 b |
| Treatment Time (min) | Treatment Voltage (kV) | ||||
|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | |
| 0 | 0.011 ± 0.001 b | 0.011 ± 0.001 b | 0.011 ± 0.001 b | 0.012 ± 0.003 b | 0.011 ± 0.001 b |
| 1 | 0.012 ± 0.003 b | 0.012 ± 0.001 ab | 0.013 ± 0.003 a | 0.012 ± 0.001 b | 0.011 ± 0.001 b |
| 2 | 0.013 ± 0.003 ab | 0.013 ± 0.003 ab | 0.011 ± 0.001 b | 0.011 ± 0.004 b | 0.012 ± 0.001 ab |
| 3 | 0.013 ± 0.003 ab | 0.014 ± 0.004 a | 0.013 ± 0.000 a | 0.013 ± 0.003 a | 0.014 ± 0.003 a |
| 4 | 0.015 ± 0.004 a | 0.012 ± 0.001 ab | 0.012 ± 0.001 b | 0.013 ± 0.003 a | 0.014 ± 0.003 a |
| Treatment Voltage (kV) | Secondary Structure of Protein (%) | |||
|---|---|---|---|---|
| α-Helix | β-Sheet | β-Turn | Random Coil | |
| 0 | 20.70 | 31.40 | 0.00 | 47.80 |
| 10 | 11.20 | 47.60 | 6.20 | 35.00 |
| 20 | 12.70 | 46.20 | 6.20 | 35.00 |
| 30 | 12.40 | 40.00 | 4.80 | 42.80 |
| 40 | 12.20 | 41.20 | 0.00 | 46.60 |
| 50 | 9.50 | 50.10 | 5.20 | 35.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Hatab, S.; Yan, J.; Miao, W.; Nyaisaba, B.M.; Piao, X.; Zheng, B.; Deng, S. Changes in Biochemical Properties and Activity of Trypsin-like Protease (Litopenaeus vannamei) Treated by Atmospheric Cold Plasma (ACP). Foods 2022, 11, 1277. https://doi.org/10.3390/foods11091277
Tang L, Hatab S, Yan J, Miao W, Nyaisaba BM, Piao X, Zheng B, Deng S. Changes in Biochemical Properties and Activity of Trypsin-like Protease (Litopenaeus vannamei) Treated by Atmospheric Cold Plasma (ACP). Foods. 2022; 11(9):1277. https://doi.org/10.3390/foods11091277
Chicago/Turabian StyleTang, Lingling, Shaimaa Hatab, Jinhong Yan, Wenhua Miao, Bhoke Marwa Nyaisaba, Xinyue Piao, Bin Zheng, and Shanggui Deng. 2022. "Changes in Biochemical Properties and Activity of Trypsin-like Protease (Litopenaeus vannamei) Treated by Atmospheric Cold Plasma (ACP)" Foods 11, no. 9: 1277. https://doi.org/10.3390/foods11091277