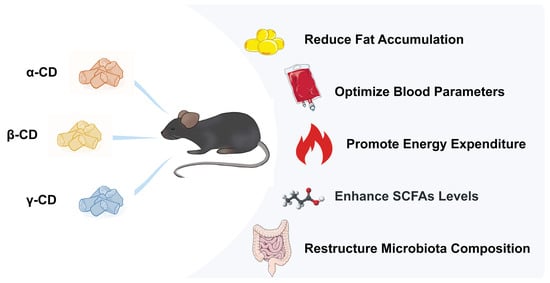
Beneficial Effects of Three Dietary Cyclodextrins on Preventing Fat Accumulation and Remodeling Gut Microbiota in Mice Fed a High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Procedures
2.3. Metabolic Rate Monitoring
2.4. Biochemical Analysis in Serum and Liver
2.5. Histopathological Analysis
2.6. Short-Chain Fatty Acid (SCFA) Analysis in Cecal Contents
2.7. S Ribosomal RNA Gene Sequencing
2.8. Quantitative RT-PCR Analysis
2.9. Statistical Analysis
3. Results
3.1. β-CD Supplementation Prevented Weight Gain and Fat Accumulation
3.2. β-CD Supplementation Optimized Blood Parameters
3.3. α-CD Supplementation Promoted Energy Expenditure and Changed the Energy Supply Structure
3.4. α-CD and γ-CD Supplementation Enhanced SCFA Levels in Cecum Contents
3.5. α-CD, β-CD, and γ-CD Supplementation Promotes Changes in Expression of Genes Involved in Lipid Metabolism
3.6. α-CD, β-CD, and γ-CD Supplementation Restructured Fecal Microbiota Compositions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piche, M.E.; Tchernof, A.; Despres, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Media Centre. Obesity and Overweight: Fact Sheet (Updated October 2017). Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 February 2022).
- Comerford, K.B.; Artiss, J.D.; Jen, K.L.C.; Karakas, S.E. The Beneficial Effects alpha-Cyclodextrin on Blood Lipids and Weight Loss in Healthy Humans. Obesity 2011, 19, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Martin del Valle, E.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J. Cyclodextrins as food ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Yan-li, W.; Chao, Y.; Ya-wei, L. Recent applications of cyclodextrins in food industry. China Food Addit. 2017, 11, 128–131. [Google Scholar]
- Van Ommen, B.; De Bie, A.; Bar, A. Disposition of C-14-alpha-cyclodextrin in germ-free and conventional rats. Regul. Toxicol. Pharm. 2004, 39, 57–66. [Google Scholar] [CrossRef]
- Nihei, N.; Okamoto, H.; Furune, T.; Ikuta, N.; Sasaki, K.; Rimbach, G.; Yoshikawa, Y.; Terao, K. Dietary alpha-cyclodextrin modifies gut microbiota and reduces fat accumulation in high-fat-diet-fed obese mice. Biofactors 2018, 44, 336–347. [Google Scholar] [CrossRef]
- Alonso, L.; Fontecha, J.; Cuesta, P.; Juarez, M.; Gilliland, S.E. Industrial application of beta-cyclodextrin for manufacturing low cholesterol butter. Milchwissenschaft 2010, 65, 36–37. [Google Scholar]
- Arora, A.; Damodaran, S. Removal of soy protein-bound phospholipids by a combination of sonication, beta-cyclodextrin, and phospholipase A2 treatments. Food Chem. 2011, 127, 1007–1013. [Google Scholar] [CrossRef]
- Ishiguro, T.; Adachi, S.; Matsuno, R. Thermogravimetric Analysis of Cyclodextrin Fatty-Acid Complex-Formation and Its Use for Predicting Suppressed Autoxidation of Fatty-Acids. Biosci. Biotech. Bioch. 1995, 59, 51–54. [Google Scholar] [CrossRef] [Green Version]
- Hamoudi, M.; Trichard, L.; Grossiord, J.L.; Chaminade, P.; Duchene, D.; Le Bas, G.; Fattal, E.; Bochot, A. Interactions between cyclodextrins and triglycerides: From emulsion stabilisation to the emergence of a new drug delivery system called “beads”. Ann. Pharm. Fr. 2009, 67, 391–398. [Google Scholar] [CrossRef]
- Ferezou, J.; Riottot, M.; Serougne, C.; Cohen-Solal, C.; Catala, I.; Alquier, C.; Parquet, M.; Juste, C.; Lafont, H.; Mathe, D.; et al. Hypocholesterolemic action of beta-cyclodextrin and its effects on cholesterol metabolism in pigs fed a cholesterol-enriched diet. J. Lipid Res. 1997, 38, 86–100. [Google Scholar] [CrossRef]
- FBCx. Available online: http://www.fbcx.com (accessed on 25 February 2022).
- Grunberger, G.; Jen, K.L.C.; Artiss, J.D. The benefits of early intervention in obese diabetic patients with FBCxTM—A new dietary fibre. Diabetes Metab. Res. Rev. 2007, 23, 56–62. [Google Scholar] [CrossRef]
- Gallaher, D.D.; Gallaher, C.M.; Plank, D.W. Alpha-cyclodextrin selectively increases fecal excretion of saturated fats. FASEB J. 2007, 21, A730. [Google Scholar] [CrossRef]
- Garcia-Mediavilla, V. Effects of dietary beta-cyclodextrin in hypercholesterolaemic rats. Pharm. Toxicol. 2003, 92, 94–99. [Google Scholar] [CrossRef]
- Harangi, J.; Beke, G.; Harangi, M.; Motyan, J.A. The digestable parent cyclodextrin. J. Incl. Phenom. Macro. Chem. 2012, 73, 335–339. [Google Scholar] [CrossRef]
- Spears, J.K.; Karr-Lilienthal, L.K.; Fahey, G.C. Influence of supplemental high molecular weight pullulan or gamma-cyclodextrin on ileal and total tract nutrient digestibility, fecal characteristics, and microbial populations in the dog. Arch. Anim. Nutr. 2005, 59, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Spears, J.K.; Karr-Lilienthal, L.K.; Grieshop, C.M.; Flickinger, E.A.; Wolf, B.W.; Fahey, G.C. Pullulans and gamma-cyclodextrin affect apparent digestibility and metabolism in healthy adult ileal cannulated dogs. J. Nutr. 2005, 135, 1946–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portune, K.J.; Benítez-Páez, A.; Del Pulgar, E.M.G.; Cerrudo, V.; Sanz, Y. Gut microbiota, diet, and obesity-related disorders-The good, the bad, and the future challenges. Mol. Nutr. Food Res. 2016, 61, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Nicolucci, A.C.; Hume, M.P.; Martinez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Fan, X.; Teng, C.; Li, Y.; Everaert, N.; Blecker, C. The Beneficial Effect of Coarse Cereals on Chronic Diseases through Regulating Gut Microbiota. Foods 2021, 10, 2891. [Google Scholar] [CrossRef]
- Liu, P.; Zhou, W.; Xu, W.; Peng, Y.; Yan, Y.; Lu, L.; Mi, J.; Zeng, X.; Cao, Y. The Main Anthocyanin Monomer from Lycium ruthenicum Murray Fruit Mediates Obesity via Modulating the Gut Microbiota and Improving the Intestinal Barrier. Foods 2021, 11, 98. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Zhao, L.P.; Zhang, F.; Ding, X.Y.; Wu, G.J.; Lam, Y.Y.; Wang, X.J.; Fu, H.Q.; Xue, X.H.; Lu, C.H.; Ma, J.L.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [Green Version]
- Arora, T.; Backhed, F. The gut microbiota and metabolic disease: Current understanding and future perspectives. J. Intern. Med. 2016, 280, 339–349. [Google Scholar] [CrossRef]
- Jia, X.; Lorenz, P.; Ballantyne, C.M. Poststatin Lipid Therapeutics: A Review. Methodist DeBakey Cardiovasc. J. 2019, 15, 32–38. [Google Scholar] [CrossRef]
- Artiss, J.D.; Brogan, K.; Brucal, M.; Moghaddam, M.; Jen, K.L. The effects of a new soluble dietary fiber on weight gain and selected blood parameters in rats. Metabolism 2006, 55, 195–202. [Google Scholar] [CrossRef]
- Levrat, M.A.; Favier, M.L.; Moundras, C.; Remesy, C.; Demigne, C.; Morand, C. Role of Dietary Propionic-Acid and Bile-Acid Excretion in the Hypocholesterolemic Effects of Oligosaccharides in Rats. J. Nutr. 1994, 124, 531–538. [Google Scholar] [CrossRef]
- Park, B.S.; Jang, A. Effects of dietary beta-cyclodextrin on plasma lipid and tissue cholesterol content in swine. Asian Austral. J. Anim. 2007, 20, 100–105. [Google Scholar] [CrossRef]
- Shimizu, I.; Aprahamian, T.; Kikuchi, R.; Shimizu, A.; Papanicolaou, K.N.; MacLauchlan, S.; Maruyama, S.; Walsh, K. Vascular rarefaction mediates whitening of brown fat in obesity. J. Clin. Investig. 2014, 124, 2099–2112. [Google Scholar] [CrossRef] [Green Version]
- Chechi, K.; Carpentier, A.C.; Richard, D. Understanding the brown adipocyte as a contributor to energy homeostasis. Trends Endocrinol. Metab. 2013, 24, 408–420. [Google Scholar] [CrossRef]
- Wagner, E.M.A.; Jen, K.-L.C.; Artiss, J.D.; Remaley, A.T. Dietary alpha-cyclodextrin lowers low-density lipoprotein cholesterol and alters plasma fatty acid profile in low-density lipoprotein receptor knockout mice on a high-fat diet. Metabolism 2008, 57, 1046–1051. [Google Scholar] [CrossRef] [Green Version]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef]
- Fernandes, J.; Su, W.; Rahat-Rozenbloom, S.; Wolever, T.M.S.; Comelli, E.M. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr. Diabetes 2014, 4, e121. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schafer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Fujisawa, K.; Ito, E.; Idei, S.; Kawaguchi, N.; Kimoto, M.; Hiemori, M.; Tsuji, H. Improvement of obesity and glucose tolerance by acetate in Type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci. Biotechnol. Biochem. 2007, 71, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremaroli, V.; Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Hartstra, A.V.; Bouter, K.E.C.; Backhed, F.; Nieuwdorp, M. Insights Into the Role of the Microbiome in Obesity and Type 2 Diabetes. Diabetes Care 2015, 38, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017, 26, 672–685.e4. [Google Scholar] [CrossRef] [Green Version]
- Asp, M.L.; Hertzler, S.R.; Chow, J.; Wolf, B.W. Gamma-cyclodextrin lowers postprandial glycemia and insulinemia without carbohydrate malabsorption in healthy adults. J. Am. Coll. Nutr. 2006, 25, 49–55. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, S.; Gan, R.Y.; Zhou, T.; Xu, D.P.; Li, H.B. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Guo, C.F.; Li, J.Y. Hypocholesterolaemic action of Lactobacillus casei F0822 in rats fed a cholesterol-enriched diet. Int. Dairy J. 2013, 32, 144–149. [Google Scholar] [CrossRef]
- Zhao, X.; Zhong, X.; Liu, X.; Wang, X.; Gao, X. Therapeutic and Improving Function of Lactobacilli in the Prevention and Treatment of Cardiovascular-Related Diseases: A Novel Perspective From Gut Microbiota. Front. Nutr. 2021, 8, 693412. [Google Scholar] [CrossRef]
- Cani, P.D.; de Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, N.; Tan, H.Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia muciniphila in Obesity: Interactions With Lipid Metabolism, Immune Response and Gut Systems. Front. Microbiol. 2020, 11, 219. [Google Scholar] [CrossRef] [Green Version]
- Eeckhaut, V.; Machiels, K.; Perrier, C.; Romero, C.; Maes, S.; Flahou, B.; Steppe, M.; Haesebrouck, F.; Sas, B.; Ducatelle, R.; et al. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut 2013, 62, 1745–1752. [Google Scholar] [CrossRef]
- Fenyvesi, E.; Vikmon, M.; Szente, L. Cyclodextrins in Food Technology and Human Nutrition: Benefits and Limitations. Crit. Rev. Food Sci. Nutr. 2016, 56, 1981–2004. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Anusree, M.; Kajal, C. Unprecedented antioxidative and anti-inflammatory aryl polyketides from the brown seaweed Sargassum wightii. Food Res. Int. 2017, 100, 640–649. [Google Scholar] [CrossRef]












| Target Gene | Sequences (5′–3′) |
|---|---|
| β-actin | F: GGGTCAGAAGGACTCCTATG R: GTAACAATGCCATGTTCAAT |
| Srebp-1c | F: AACTTTTCCTTAACGTGGGCCT R: TGTCCAGTTCGCACATCTCG |
| Pparα | F: ACTACGGAGTTCACGCATGTG R: TTGTCGTACACCAGCTTCAGC |
| Pepck | F: TCTTTGGTGGCCGTAGACCTG R: CCAGGTATTTGCCGAAGTTGTAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, T.; Zhang, B.; Feng, Y.; Li, Z.; Tang, X.; Ban, X.; Kong, H.; Li, C. Beneficial Effects of Three Dietary Cyclodextrins on Preventing Fat Accumulation and Remodeling Gut Microbiota in Mice Fed a High-Fat Diet. Foods 2022, 11, 1118. https://doi.org/10.3390/foods11081118
Zhu T, Zhang B, Feng Y, Li Z, Tang X, Ban X, Kong H, Li C. Beneficial Effects of Three Dietary Cyclodextrins on Preventing Fat Accumulation and Remodeling Gut Microbiota in Mice Fed a High-Fat Diet. Foods. 2022; 11(8):1118. https://doi.org/10.3390/foods11081118
Chicago/Turabian StyleZhu, Tong, Baixi Zhang, Yan Feng, Zhaofeng Li, Xiaoshu Tang, Xiaofeng Ban, Haocun Kong, and Caiming Li. 2022. "Beneficial Effects of Three Dietary Cyclodextrins on Preventing Fat Accumulation and Remodeling Gut Microbiota in Mice Fed a High-Fat Diet" Foods 11, no. 8: 1118. https://doi.org/10.3390/foods11081118