Genetic Engineering Production of Ethyl Carbamate Hydrolase and Its Application in Degrading Ethyl Carbamate in Chinese Liquor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Chemical Reagents
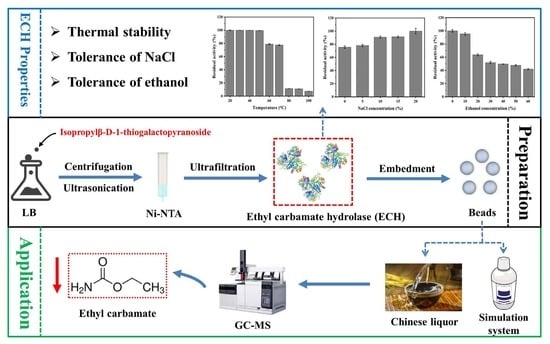
2.2. Construction of Recombinant Escherichia coli (E. coli) for Overexpression of ECH
2.3. Expression of ECH in E. coli BL21 (DE3)
2.4. Purification of Recombinant ECH and SDS-PAGE Analysis
2.5. Recombinant ECH Activity Assays
2.6. The Enzymatic Properties of Recombinant ECH
2.6.1. The Optimum Temperature of Recombinant ECH
2.6.2. The Thermal Stability of Recombinant ECH
2.6.3. The Optimum pH of Recombinant ECH
2.6.4. Effect of NaCl Concentration and Ethanol Concentration on Recombinant ECH Activity
2.6.5. Effect of EDTA and Metal Ions on Recombinant ECH Activity
2.6.6. Substrate Specificity of Recombinant ECH
2.6.7. Determination of Kinetic Parameters of an Enzyme-Catalyzed Reaction
2.7. Immobilized Recombinant ECH Preparation
2.8. The Degradation of EC in Simulation System by Recombinant ECH
2.9. The Degradation of EC in Chinese Liquor by Immobilized ECH
2.10. Determination of Volatile Organic Compounds in Chinese Liquor
2.11. Statistical Analysis
3. Results and Discussion
3.1. Expression of Recombinant ECH in E. coli BL21 (DE3)
3.2. Biochemical Characterization of ECH
3.3. The Degradation of EC with Immobilized ECH in Simulation System
3.4. Use ECH to Remove EC in Chinese Liquor
3.5. Effect of ECH on Flavor Substances in Chinese Liquor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiao, Z.; Dong, Y.; Chen, Q. Ethyl Carbamate in Fermented Beverages: Presence, Analytical Chemistry, Formation Mechanism, and Mitigation Proposals. In Comprehensive Reviews in Food Science and Food Safety; Blackwell Publishing Inc.: Oxford, UK, 2014; pp. 611–626. [Google Scholar] [CrossRef]
- Field, K.J.; Lang, C.M. Hazards of Urethane (Ethyl Carbamate): A Review of the Literature. Lab. Anim. 1988, 22, 255–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.-K.; Liem, A.; Stewart, B.C.; Miller, J.A. Vinyl Carbamate Epoxide, a Major Strong Electrophilic, Mutagenic and Carcinogenic Metabolite of Vinyl Carbamate and Ethyl Carbamate (Urethane); Wolters Kluwer–Medknow Publications Wolters Kluwer India Pvt. Ltd.: Mumbai, India, 1993; Volume 14. [Google Scholar]
- Sakano, K.; Oikawa, S.; Hiraku, Y.; Kawanishi, S. Original Contribution Metabolism of Carcinogenic Urethane to Nitric Oxide Is Involved in Oxidative Dna Damage; Elsevier Science Publishing Company Inc.: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Zhao, X.; Du, G.; Zou, H.; Fu, J.; Zhou, J.; Chen, J. Progress in preventing the accumulation of ethyl carbamate in alcoholic beverages. Trends Food Sci. Technol. 2013, 32, 97–107. [Google Scholar] [CrossRef]
- Akinyanju, J.A.; Oyedeji, B.M. Comparative Study of Mixed Flora and Single Strain Fermentation in the Production of Plantain (Musa Paradisiaca) “Wine”. Chem. Mikrobiol. Technol. Lebensm. 1993, 15, 975–977. [Google Scholar]
- Wu, D.; Li, X.; Shen, C.; Lu, J.; Chen, J.; Xie, G. Decreased Ethyl Carbamate Generation during Chinese Rice Wine Fermentation by Disruption of CAR1 in an Industrial Yeast Strain. Int. J. Food Microbiol. 2014, 180, 19–23. [Google Scholar] [CrossRef]
- Guo, X.W.; Li, Y.Z.; Guo, J.; Wang, Q.; Huang, S.Y.; Chen, Y.F.; Du, L.P.; Xiao, D.G. Reduced Production of Ethyl Carbamate for Wine Fermentation by Deleting CAR1 in Saccharomyces Cerevisiae. J. Ind. Microbiol. Biotechnol. 2016, 43, 671–679. [Google Scholar] [CrossRef]
- Wu, D.; Li, X.; Lu, J.; Chen, J.; Zhang, L.; Xie, G. Constitutive Expression of the DUR1,2 Gene in an Industrial Yeast Strain to Minimize Ethyl Carbamate Production during Chinese Rice Wine Fermentation. FEMS Microbiol. Lett. 2015, 363, fnv214. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Xie, W.; Li, X.; Cai, G.; Lu, J.; Xie, G. Metabolic Engineering of Saccharomyces Cerevisiae Using the CRISPR/Cas9 System to Minimize Ethyl Carbamate Accumulation during Chinese Rice Wine Fermentation. Appl. Microbiol. Biotechnol. 2020, 104, 4435–4444. [Google Scholar] [CrossRef]
- Moreau, M.-C.; Ducluzeau, R.; Raibaud, P. Hydrolysis of Urea in the Gastrointestinal Tract of “Monoxenic” Rats: Effect of Immunization with Strains of Ureolytic Bacteria; American Society for Microbiology: Washington, DC, USA, 1976; Volume 13. [Google Scholar]
- Suzuki, K.; Benno, Y.; Mitsuoka, T.; Takebe, S.; Kobashi, K.; Hase, J. Urease-Producing Species of Intestinal Anaerobes and Their Activities; American Society for Microbiology: Washington, DC, USA, 1979; Volume 37. [Google Scholar]
- Yang, L.Q.; Wang, S.H.; Tian, Y.P. Purification, Properties, and Application of a Novel Acid Urease from Enterobacter sp. Appl. Biochem. Biotechnol. 2010, 160, 303–313. [Google Scholar] [CrossRef]
- Mora, D.; Fortina, M.G.; Parini, C.; Ricci, G.; Gatti, M.; Giraffa, G.; Manachini, P.L. Genetic Diversity and Technological Properties of Streptococcus Thermophilus Strains Isolated from Dairy Products. J. Appl. Microbiol. 2002, 93, 278–287. [Google Scholar] [CrossRef]
- Mora, D.; Maguin, E.; Masiero, M.; Parini, C.; Ricci, G.; Manachini, P.L.; Daffonchio, D. Characterization of Urease Genes Cluster of Streptococcus Thermophilus. J. Appl. Microbiol. 2004, 96, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Mora, D.; Monnet, C.; Parini, C.; Guglielmetti, S.; Mariani, A.; Pintus, P.; Molinari, F.; Daffonchio, D.; Manachini, P.L. Urease Biogenesis in Streptococcus Thermophilus. Res. Microbiol. 2005, 156, 897–903. [Google Scholar] [CrossRef]
- Zotta, T.; Ricciardi, A.; Rossano, R.; Parente, E. Urease Production by Streptococcus Thermophilus. Food Microbiol. 2008, 25, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, S.; Okazaki, K.; Sakane, T.; Imai, K.; Sumino, Y.; Akiyama, S.I.; Nakao, Y. Isolation and Taxonomic Characterization of Acid Ureaseproducing Bacteria. Agric. Biol. Chem. 1989, 53, 1111–1117. [Google Scholar] [CrossRef]
- Chen, Y.-Y.M.; Clancy, K.A.; Burne, R.A. Streptococcus Salivarius Urease: Genetic and Biochemical Characterization and Expression in a Dental Plaque Streptococcus. Infect. Immun. 1996, 64, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Kang, Z.; Liu, Q.; Du, G.; Chen, J. Degradation of Urea and Ethyl Carbamate in Chinese Rice Wine by Recombinant Acid Urease. Chin. J. Biotechnol. 2016, 32, 74–83. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Ethyl Carbamate and Hydrocyanic Acid in Food and Beverages—Scientific Opinion of the Panel on Contaminants. EFSA J. 2007, 551, 1–44. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Lima, M.C.P.; Nóbrega, I.C.C.; Pereira, J.A.P.; Kerr-Corrêa, F.; Kanteres, F.; Rehm, J. Cancer Risk Assessment of Ethyl Carbamate in Alcoholic Beverages from Brazil with Special Consideration to the Spirits Cachaça and Tiquira. BMC Cancer 2010, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Pan, X.; Wang, L.; Shen, X.; Yang, D. A Survey of Ethyl Carbamate in Fermented Foods and Beverages from Zhejiang, China. Food Control 2012, 23, 286–288. [Google Scholar] [CrossRef]
- Yoshida, K.; Takamasa, T. Decomposition of Urethane. Patent CN111065280A, 24 April 2020. [Google Scholar]
- Wang, J.; Zhang, S.; Tan, H.; Zhao, Z. PCR-Based Strategy for Construction of Multi-Site-Saturation Mutagenic Expression Library. J. Microbiol. Methods 2007, 71, 225–230. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Y.; Yuan, M.; Du, G.; Chen, J.; Kang, Z. A Bacillus Paralicheniformis Ironcontaining Urease Reduces Urea Concentrations in Rice Wine. Appl. Environ. Microbiol. 2017, 83, e01258-17. [Google Scholar] [CrossRef] [Green Version]
- Kang, T.; Lin, J.; Yang, L.; Wu, M. Expression, Isolation, and Identification of an Ethanol-Resistant Ethyl Carbamate-Degrading Amidase from Agrobacterium Tumefaciens D3. J. Biosci. Bioeng. 2021, 132, 220–225. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, Y.; Wang, D.; Xu, Y. Immobilized Rhodotorula Mucilaginosa: A Novel Urethanase-Producing Strain for Degrading Ethyl Carbamate. Appl. Biochem. Biotechnol. 2013, 171, 2220–2232. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Wu, Q.; Xu, Y. Biodegradation of Ethyl Carbamate and Urea with Lysinibacillus Sphaericus MT33 in Chinese Liquor Fermentation. J. Agric. Food Chem. 2018, 66, 1583–1590. [Google Scholar] [CrossRef]
- Xia, Q.; Yuan, H.; Wu, C.; Zheng, J.; Zhang, S.; Shen, C.; Yi, B.; Zhou, R. An Improved and Validated Sample Cleanup Method for Analysis of Ethyl Carbamate in Chinese Liquor. J. Food Sci. 2014, 79, T1854–T1860. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; He, Z.; Wang, X.; Song, G.; Chen, H.; Lin, X.; Ji, C.; Zhang, S. Bacterial Profiles and Volatile Flavor Compounds in Commercial Suancai with Varying Salt Concentration from Northeastern China. Food Res. Int. 2020, 137, 109384. [Google Scholar] [CrossRef]
- Crowe, J.; Döbeli, H.; Gentz, R.; Hochuli, E.; Stiiber, D.; Henco, K. 6xffis-Ni-NTA Chromatography as a Superior Technique in Recombinant Protein Expression/Purification. Protoc. Gene Anal. 1994, 31, 371–387. [Google Scholar] [CrossRef]
- Zhao, C.; Kobashi, K. Purification and Characterization of Iron-Containing Urethanase from Bacillus licheniformis. Biol. Pharm. Bull. 1994, 17, 773–778. [Google Scholar] [CrossRef] [Green Version]
- Akutsu-Shigeno, Y.; Adachi, Y.; Yamada, C.; Toyoshima, K.; Nomura, N.; Uchiyama, H.; Nakajima-Kambe, T. Isolation of a Bacterium That Degrades Urethane Compounds and Characterization of Its Urethane Hydrolase. Appl. Microbiol. Biotechnol. 2006, 70, 422–429. [Google Scholar] [CrossRef]
- Kobashi, K.; Takebe, S.; Sakai, T. Urethane-hydrolyzing enzyme from Citrobacter sp. Chem. Pharm. Bull. 1990, 38, 1326–1328. [Google Scholar] [CrossRef] [Green Version]
- Zhou, N.-D.; Gu, X.-L.; Tian, Y.-P. Isolation and Characterization of Urethanase from Penicillium variabile and Its Application to Reduce Ethyl Carbamate Contamination in Chinese Rice Wine. Appl. Biochem. Biotechnol. 2013, 170, 718–728. [Google Scholar] [CrossRef]
- Jianqing, D. The Process Optimization of Maotai Liquor Fortified High Temperature Daqu and Its Comparative Research; Fujian Normal University: Fuzhou, China, 2017. [Google Scholar]
- Masaki, K.; Fujihara, K.; Kakizono, D.; Mizukure, T.; Okuda, M.; Mukai, N. Aspergillus Oryzae Acetamidase Catalyzes Degradation of Ethyl Carbamate. J. Biosci. Bioeng. 2020, 130, 577–581. [Google Scholar] [CrossRef]
- Liu, X.; Fang, F.; Xia, X.; Du, G.; Chen, J. Stability Enhancement of Urethanase from Lysinibacillus Fusiformis by Site-Directed Mutagenesis. Chin. J. Biotechnol. 2016, 32, 1233–1242. [Google Scholar] [CrossRef]
- Bu, P.; Chen, J.; Du, G. Purification and Characterization of a Halophilic Urethanase from Klebsiella Pneumoniae. Chin. J. Biotechnol. 2014, 30, 404–411. [Google Scholar] [CrossRef]
- Xia, D.; QiaoYu, L.; Fang, L.; WeiZhu, Z.; Jian, C.; GuoCheng, D.; Fang, F. Isolation of Microbial Strains for Degradation of Etliyl Carbamate in Luzhouflavour Baijiu and Characterization of Corresponding Enzymes. Food Ferment. Ind. 2018, 44, 29–36. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, S.; Li, Y.; Xie, C.; Li, H.; Ding, X. A Chinese Liquor Classification Method Based on Liquid Evaporation with One Unmodulated Metal Oxide Gas Sensor. Sens. Actuators B Chem. 2011, 160, 483–489. [Google Scholar] [CrossRef]
- Liu, Q.; Yao, X.; Liang, Q.; Li, J.; Fang, F.; Du, G.; Kang, Z. Molecular Engineering of Bacillus Paralicheniformis Acid Urease to Degrade Urea and Ethyl Carbamate in Model Chinese Rice Wine. J. Agric. Food Chem. 2018, 66, 13011–13019. [Google Scholar] [CrossRef]
- van Gemert, L.J. Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners BV, The Netherlands: Utrecht, The Netherlands, 2011. [Google Scholar]




| Metal Ion Chemical | Relative Activity (%) | Standard Deviation (SD) |
|---|---|---|
| Zn2+ | 56.71 | 1.92 |
| Mg2+ | 64.99 | 2.55 |
| Ca2+ | 58.48 | 1.54 |
| Mn2+ | 96.80 | 2.42 |
| Fe2+ | 58.66 | 5.22 |
| Fe3+ | 10.32 | 1.63 |
| EDTA | 35.40 | 4.78 |
| Substrate | Relative Activity (%) | SD |
|---|---|---|
| Methyl carbamate | 132.04 | 0.57 |
| Ethyl carbamate | 100.00 | 1.30 |
| Butyl Carbamate | 80.34 | 0.78 |
| Urea | 0.00 | 1.87 |
| Compounds | Concentration (mg/L) | ||
|---|---|---|---|
| Control | 12 h | ||
| Esters | ethyl acetate | 13.63 ± 1.10 | 7.23 ± 0.91 |
| butanoic acid, ethyl ester | 29.45 ± 0.52 | 19.20 ± 0.33 | |
| pentanoic acid, ethyl ester | 22.18 ± 2.21 | 13.51 ± 1.26 | |
| hexanoic acid, ethyl ester | 490.58 ± 16.74 | 382.48 ± 5.27 | |
| hexanoic acid, propyl ester | 5.248 ± 0.10 | 4.27 ± 1.26 | |
| heptanoic acid, ethyl ester | 35.14 ± 0.87 | 31.61 ± 0.94 | |
| hexanoic acid, butyl ester | 20.42 ± 0.28 | 20.34 ± 0.50 | |
| octanoic acid, ethyl ester | 58.77 ± 1.32 | 58.62 ± 1.15 | |
| isopentylhexanoate | 13.31 ± 0.21 | 15.18 ± 0.30 | |
| hexanoic acid, pentyl ester | 9.06 ± 0.25 | 11.00 ± 0.14 | |
| nonanoic acid, ehtyl ester | 2.74 ± 0.04 | 4.37 ± 0.04 | |
| hexanoic acid, hexyl ester | 37.67 ± 0.92 | 50.39 ± 0.97 | |
| decanoic acid, ethyl ester | 6.69 ± 0.19 | 13.27 ± 0.27 | |
| heptanoic acid, heptyl ester | 1.53 ± 0.04 | 4.28 ± 0.16 | |
| octanoic acid, hexyl ester | 1.35 ± 0.01 | 4.43 ± 0.07 | |
| tetradecanoic acid, ethyl ester | 2.20 ± 0.12 | 12.31 ± 0.66 | |
| hexadecanoic acid, ethyl ester | 7.71 ± 2.22 | 33.82 ± 1.41 | |
| 9-octadecenoic acid (z)-, eicosyl ester | 0.48 ± 0.05 | 6.74 ± 0.21 | |
| ethyl iso-allocholate | 0.27 ± 0.05 | 4.02 ± 0.38 | |
| Aromatic | benzeneacetic acid, ethyl ester | 3.44 ± 0.09 | 4.38 ± 0.08 |
| benzenepropanoic acid, ethyl ester | 2.43 ± 0.13 | 6.77 ± 4.86 | |
| Alcohol | 1-pentanol | 3.06 ± 0.18 | 3.85 ± 0.27 |
| Aldehyde/furan | furfural | 13.92 ± 0.25 | 8.56 ± 0.25 |
| Organic acid | hexanoic acid | 29.20 ± 0.82 | 28.51 ± 0.13 |
| Other | butane,1,1-diethoxy-3-methyl- | 0.69 ± 0.41 | 0.78 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, N.; Xue, S.; Guo, H.; Xiong, K.; Lin, X.; Liang, H.; Ji, C.; Huang, Z.; Zhang, S. Genetic Engineering Production of Ethyl Carbamate Hydrolase and Its Application in Degrading Ethyl Carbamate in Chinese Liquor. Foods 2022, 11, 937. https://doi.org/10.3390/foods11070937
Dong N, Xue S, Guo H, Xiong K, Lin X, Liang H, Ji C, Huang Z, Zhang S. Genetic Engineering Production of Ethyl Carbamate Hydrolase and Its Application in Degrading Ethyl Carbamate in Chinese Liquor. Foods. 2022; 11(7):937. https://doi.org/10.3390/foods11070937
Chicago/Turabian StyleDong, Naihui, Siyu Xue, Hui Guo, Kexin Xiong, Xinping Lin, Huipeng Liang, Chaofan Ji, Zhiguo Huang, and Sufang Zhang. 2022. "Genetic Engineering Production of Ethyl Carbamate Hydrolase and Its Application in Degrading Ethyl Carbamate in Chinese Liquor" Foods 11, no. 7: 937. https://doi.org/10.3390/foods11070937