1. Introduction
Over the past years, advances in neonatal intensive care have improved the survival of very-low-birth-weight neonates [
1]. Feeding with pasteurized banked milk (PBM) has been regarded as an essential part of neonatal intensive care as it supports neurocognitive development [
2,
3], reduces the risk of obesity in later life [
4], and protects cardiovascular health in adulthood [
5]. Lowering the incidence of necrotizing enterocolitis in newborns fed PBM compared to that in babies fed milk formula is a significant advantage for this type of feeding [
6]. On the other hand, due to the immaturity of the immune system in extremely-low-birth-weight neonates, they are at risk of opportunistic infections, such as
B. cereus [
7,
8,
9,
10,
11,
12]. While in healthy newborns, feeding with PBM is temporary support in cases of the mother’s delayed or insufficient lactation, in very-low-birth-weight neonates, PBM represents the only option because breastfeeding is not possible. Preferably the PBM used is expressed by their mothers, with donated PBM being the second option. Standard Holder pasteurization followed by rapid chilling and/or freezing of human milk is recommended by the EMBA, which also recommends routine post-pasteurization bacteriological analysis [
13]. Such analysis is not yet obligatory in the Czech Republic [
14], nevertheless, Czech Human Milk Banks are obliged to perform regular evaluations of their pasteurization process as a part of the Hazard Analysis and Critical Points system (HACCP) and to perform corrective actions, as necessary [
15]. Many human milk banks perform pre-pasteurization pooling of milk and take a post-pasteurization sample representing a large volume of the pooled milk [
7,
16]. The quality assurance system of our Human Milk Bank is based on avoidance of pooling and taking samples from each pasteurized bottle with a volume of 250 mL [
15].
To facilitate risk evaluation, the predictive microbiology databases COMBASE and Pathogen Modeling Program are available. Lewin et al., 2020 [
16] used a different prediction approach based on the Monte Carlo method using statistical data from disease cases and determined the probability of infection per 1 million doses of PBM. Lewin’s simulation study [
16] remained limited to the safety of PBM at the moment of its release for distribution. An evaluation of
B. cereus growth curves in milk products under different conditions based on experimental data and the COMBASE Predictive Models was published by Soleimaninanadegani [
17]. Results of mathematical modeling of
B. cereus growth in milk were also published by Teleken et al. [
18], Ačai et al. [
19], Larsen et al. [
20], and Hyoun Wook, K. et al. [
21]. They published predictive models to evaluate the kinetic behaviors of
Bacillus cereus and
Staphylococcus aureus in milk during storage at various temperatures.
In our previous retrospective study based on data from 2017–2020, we identified
B. cereus as the most common cause of post-pasteurization PBM discard in our Human Milk Bank [
15]. As extension of the previous investigation, we deal with assessing the residual risk that may occur during the warming of the thawed PBM and during temporary storage in the warmed state prior to administration to newborns at neonatal intensive care units (NICU).
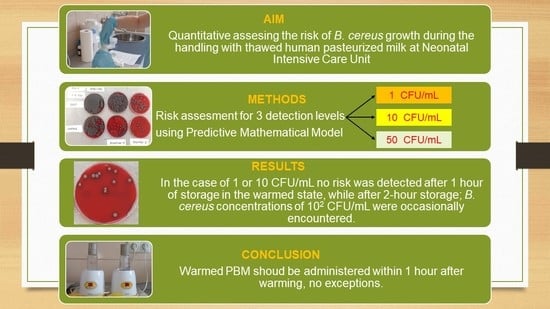
In this paper, we used a predictive mathematical model created on real temperature history data, water activity, and
B. cereus growth in artificially inoculated PBM to assess this risk. The risk was assessed for three cases: 1. An initial post-pasteurization
B. cereus concentration of 1 colony-forming unit (CFU)/mL, which is equal to the level of detection; 2. a
B. cereus concentration of 10 CFU/mL, which is allowed in some countries [
13]; and 3. a
B.cereus concentration of 50 CFU/mL, which is approved for milk formulas [
22,
23]. The actual risk level was set at 100 CFU of
B. cereus/mL.
As some authors state, precise quantification of risks is more important than in the context of food manufacturing alone [
24]. The originality of our work is in quantitative risk assessment of
B. cereus growth in thawed human pasteurized milk during manipulation at neonatal units of Pediatric departments.
4. Discussion
The traditional approach to evaluating the quality and microbiological safety of food products is based on a comparison of results of the bacteriological output analysis with limits set by the manufacturer, published data of manufacturers of similar products, and assessment of the compliance rate with existing national or international norms or recommendations. Such an approach was partly applied in our previous paper in which we compared the post-pasteurization discard rate with similar data published by French Human Milk Banks (HMBs) [
15,
22,
28]. Such a comparison can provide relevant data only when similar processing and analysis methods are used. From this point of view, our data compares well with HMBs that use standard Holder pasteurization and MALDI mass spectrometry for post-pasteurization microbiological analysis. Cormontagne [
22] reported that PBM discard rates, after introducing the MALDI method, fluctuated between 19.0 and 21.2%, while our discard rate fluctuated between 8.6 and 10.5%, with
B. cereus being responsible for 67.24% of the discard. Cormontagne [
22] reported that as much as 90% of PBM discard was caused by
B. cereus. Noncompliance rates as high as 27.3%, mainly caused by
B. cereus, were reported by Adjidé, 2019 [
29]. Lewin, 2019 reported that the discard rates between 25 and 35% were typical in Canadian Human Milk Banks, and the proportion caused by
B. cereus was between 80% and 90% [
16]. Mallardi recently published a review of complex measures that substantially lowered the PBM discard rate from 19.5% to 14.3% [
30].
Another critical factor is the manufacturer’s post-pasteurization discard limit. A limit of below 1 CFU/mL was used in our previous paper [
15] and was equal to the limit published by Cormontagne [
22], Adjidé [
29], and Lewin [
16]. Using this limit, Lewin predicted that the real
B. cereus concentration fluctuates between 0.21 and 0.64 CFU/mL.
The results of quantitative post-pasteurization analysis published previously [
15] were used for comparison with different
B. cereus contamination limits included in HMBs standards or food norms.
Table 9 shows 80% compliance of these samples with existing HMB microbiological safety post-pasteurization standards and 90% compliance with the norms set for food used for children under six months (
Table 9). These data document the high microbiological safety of our PBM at the moment of delivery from the HMB.
Another approach to evaluating microbiological safety uses the limits for the total number of ingested
B. cereus CFUs reported as dangerous. A range for the
B. cereus ingested load of between 10
5 and 10
8 CFUs was presented by Vidic [
31], while other sources [
32] regard amounts as low as 10
3 to 10
4 as hazardous. A similar approach based on determining the maximum number of
B. cereus CFUs ingested per one feeding was also applied in the simulation study presented by Lewin [
11]. Other authors report dangerous concentrations of
B. cereus CFUs per 1 g of food. Bacterial counts of 10
5 to 10
8 CFU/g of food can generate disease-relevant amounts of toxins in foods or the small intestine [
33]. Most food-borne outbreaks caused by the
B. cereus group have been associated with bacterial concentrations above 10
5 CFU/g of foodstuff. However, it is important to highlight that both emetic and diarrheal disease have been reported for
B. cereus counts of 10
3 to 10
5 CFU/g [
34].
There is a consensus that even post-pasteurization microbiological negativity of PBM does not eliminate the risk of its use. During feeding of newborns, the above-presented data of B. cereus concentration and/or of the total amount of CFUs ingested should not be exceeded.
The methods of predictive microbiology are generally regarded to be a standard tool for assessing the risk of food, the properties of which are changing over time because of the presence of viable bacteria. We used this method in the past for predicting the growth of several microbes, including
B. cereus, during the processing of human milk [
35] and for evaluating thermo- and baro-inactivation of
E. faecium and
St. epidermidis in human and cow milk [
36,
37]. This paper applies this method to simulate manipulations with thawed PBM in neonatal hospital wards.
The presented prediction showed different results when the maximal acceptable
B. cereus concentration of 100 CFU/mL was used (
Table 5,
Table 6,
Table 7 and
Table 8), or if the total number of 1000 CFU/mL of
B. cereus ingested per day was used as the acceptable upper limit (
Table 6 and
Table 8) (the total
B. cereus CFU numbers ingested per day shown in
Table 6 and
Table 8 were calculated for a total amount of 80 mL of PBM used daily for the feeding of premature newborns). Based on the
B. cereus CFU/g limit, the first approach showed a relatively wide range of safe initial
B. cereus CFU post-pasteurization concentrations and warming times. When using our SOP discard criteria (
Table 9) and our instructions to use PBM within 1 h of thawing [
15] (
Table 5), this limit was not exceeded even after 2 h of warming (
Table 6); moreover,
Table 7 demonstrated that even PBM with initial post-pasteurization
B. cereus concentrations of 10, 50, and 100 CFU/mL PBM used within 1 h of warming could be considered relatively safe; however, 2 or 3 h of warming PBM with these initial
B. cereus CFU post-pasteurization concentrations, which are compliant with the general food standards (
Table 9), should not be considered safe.
When using the second approach, i.e., the total limit of
B. cereus CFU ingested per day (
Table 6 and
Table 8), the range of both safe initial
B. cereus CFU/mL concentrations and warming times were much narrower.
PBM compliant with our SOP was again proven safe if used within 1 h of warming. After 2 h, the total
B. cereus CFU limit could occasionally be exceeded (
Table 6). In case of using the PBM with an initial concentration of 10 CFU/mL, marginal total
B. cereus CFU numbers could be expected after one hour of warming as shown in
Table 8. PBM with initial concentrations of 50 CFU/mL, even if used within 1 h, produced unacceptable total CFU counts (
Table 8).
Our calculations probably overestimated the risk for three reasons. The first is that the risk was calculated for a 100% proportion of
B. cereus in the total PBM discard, while in reality, this proportion can vary between 70% [
15] and 90% [
22]. The second reason is our predictive mathematical model’s wide uncertainty limit range (
Table 5,
Table 6,
Table 7 and
Table 8). The third reason may be a prolonged lag phase in microbes damaged by the processing techniques described in our previous paper, i.e., pasteurization followed by rapid chilling and freezing [
15]. This is supported by
B. cereus growth diagrams (
Figures S5–S7 in the supplementary data). Nevertheless, the results of the presented predictions (
Table 5 and
Table 6) support the current practice of HMBs and their post-pasteurization discard limits [
13] (
Table 9) and confirm the high level of safety of our past and current practices [
15]. In this study oriented to the needs of the routine practice of HMBs, we did not consider the toxinogenicity of
B. cereus isolates surviving pasteurization that was reported to occur in 15% in our previous paper [
15]. Comparison of predictions using different
B. cereus toxinogenic strains might be a subject of the future research.
Since a high proportion of PBM is used in neonatal intensive care units (
Table 3 and
Table 4), we recommend using the second approach to risk assessment based on an estimation of the total number of CFU ingested per day per newborn.