Effect of Combination of Time and Temperature on Quality Characteristics of Sous Vide Chicken Breast
Abstract
:1. Introduction
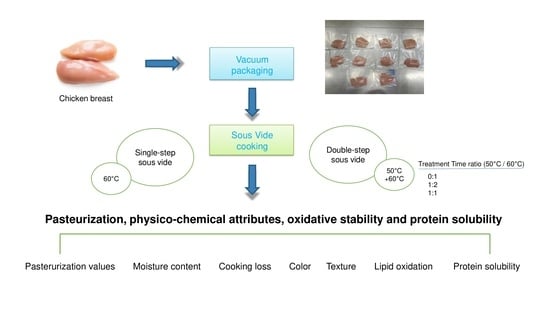
2. Materials and Methods
2.1. Experimental Design
2.2. Pasteurization Values
2.3. Physicochemical Analysis
2.3.1. Moisture Content and Cooking Loss
2.3.2. Color Measurement
2.3.3. Texture Properties
2.4. Lipid Oxidation
2.5. Protein Solubility
2.6. Statistical Analysis
3. Results
3.1. Investigation of Pasteurization Values of the Sous Vide Treatments
3.2. Physicochemical Analysis
3.2.1. Moisture Content and Cooking Loss
3.2.2. Color Measurement
3.2.3. Texture Properties
3.3. Lipid Oxidation
3.4. Protein Solubility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marangoni, F.; Corsello, G.; Cricelli, C.; Ferrara, N.; Ghiselli, A.; Lucchin, L.; Poli, A. Role of poultry meat in a balanced diet aimed at maintaining health and wellbeing: An Italian consensus document. Food Nutr. Res. 2015, 59, 27606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donma, M.M.; Donma, O. Beneficial effects of poultry meat consumption on cardiovascular health and the prevention of childhood obesity. Med. One 2017, 2, e170018. [Google Scholar] [CrossRef]
- Rémond, D.; Machebeuf, M.; Yven, C.; Buffière, C.; Mioche, L.; Mosoni, L.; Mirand, P.P. Postprandial Whole-Body Protein Metabolism after a Meat Meal Is Influenced by Chewing Efficiency in Elderly Subjects. Am. J. Clin. Nutr. 2007, 85, 1286–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Weyant, R.J.; Corby, P.; Kritchevsky, S.B.; Harris, T.B.; Rooks, R.; Rubin, S.M.; Newman, A.B. Edentulism and nutritional status in a biracial sample of well-functioning, community-dwelling elderly: The health, aging, and body composition study. Am. J. Clin. Nutr. 2004, 79, 295–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Bowker, B.; Yang, Y.; Pang, B.; Zhuang, H. Effects of Deboning Time and Thawing Method Interaction on Sensory Descriptive Profiles of Cooked Chicken Breast and Thigh Meat. LWT 2020, 120, 108939. [Google Scholar] [CrossRef]
- Hong, G.-E.; Kim, J.-H.; Ahn, S.-J.; Lee, C.-H. Changes in Meat Quality Characteristics of the Sous-Vide Cooked Chicken Breast during Refrigerated Storage. Korean J. Food Sci. Anim. Resour. 2015, 35, 757–764. [Google Scholar] [CrossRef] [Green Version]
- Zavadlav, S.; Blažić, M.; Van de Velde, F.; Vignatti, C.; Fenoglio, C.; Piagentini, A.M.; Pirovani, M.E.; Perotti, C.M.; Bursać Kovačević, D.; Putnik, P. Sous-Vide as a Technique for Preparing Healthy and High-Quality Vegetable and Seafood Products. Foods 2020, 9, 1537. [Google Scholar] [CrossRef]
- Ruiz-Carrascal, J.; Roldan, M.; Refolio, F.; Perez-Palacios, T.; Antequera, T. Sous-Vide Cooking of Meat: A Maillarized Approach. Int. J. Gastron. Food Sci. 2019, 16, 100138. [Google Scholar] [CrossRef]
- Baldwin, D.E. Sous Vide Cooking: A Review. Int. J. Gastron. Food Sci. 2012, 1, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Díaz, P.; Nieto, G.; Garrido, M.D.; Bañón, S. Microbial, Physical–Chemical and Sensory Spoilage during the Refrigerated Storage of Cooked Pork Loin Processed by the Sous Vide Method. Meat Sci. 2008, 80, 287–292. [Google Scholar] [CrossRef]
- Rinaldi, M.; Dall’Asta, C.; Paciulli, M.; Cirlini, M.; Manzi, C.; Chiavaro, E. A Novel Time/Temperature Approach to Sous Vide Cooking of Beef Muscle. Food Bioprocess. Technol. 2014, 7, 2969–2977. [Google Scholar] [CrossRef]
- Botinestean, C.; Keenan, D.F.; Kerry, J.P.; Hamill, R.M. The Effect of Thermal Treatments Including Sous-Vide, Blast Freezing and Their Combinations on Beef Tenderness of M. Semitendinosus Steaks Targeted at Elderly Consumers. LWT 2016, 74, 154–159. [Google Scholar] [CrossRef]
- Mortensen, L.M.; Frøst, M.B.; Skibsted, L.H.; Risbo, J. Effect of time and temperature on sensory properties in low-temperature long-time sous-vide cooking of beef. J. Culin. Sci. Technol. 2012, 10, 75–90. [Google Scholar] [CrossRef]
- Christensen, L.; Ertbjerg, P.; Aaslyng, M.D.; Christensen, M. Effect of Prolonged Heat Treatment from 48 °C to 63 °C on Toughness, Cooking Loss and Color of Pork. Meat Sci. 2011, 88, 280–285. [Google Scholar] [CrossRef]
- Bıyıklı, M.; Akoğlu, A.; Kurhan, Ş.; Akoğlu, İ.T. Effect of Different Sous Vide Cooking Temperature-Time Combinations on the Physicochemical, Microbiological, and Sensory Properties of Turkey Cutlet. Int. J. Gastron. Food Sci. 2020, 20, 100204. [Google Scholar] [CrossRef]
- Głuchowski, A.; Czarniecka-Skubina, E.; Buła, M. The Use of the Sous-Vide Method in the Preparation of Poultry at Home and in Catering—Protection of Nutrition Value Whether High Energy Consumption. Sustainability 2020, 12, 7606. [Google Scholar] [CrossRef]
- Karpińska-Tymoszczyk, M.; Draszanowska, A.; Danowska-Oziewicz, M.; Kurp, L. The Effect of Low-Temperature Thermal Processing on the Quality of Chicken Breast Fillets. Food Sci. Technol. Int. 2020, 26, 563–573. [Google Scholar] [CrossRef]
- Ji, D.-S.; Kim, J.-H.; Yoon, D.-K.; Kim, J.-H.; Lee, H.; Cho, W.-Y.; Lee, C.-H. Effect of Different Storage-Temperature Combinations on Longissimus Dorsi Quality upon Sous-Vide Processing of Frozen/Thawed Pork. Food Sci. Anim. Resour. 2019, 39, 240–254. [Google Scholar] [CrossRef]
- Ashie, I.N.A.; Sorensen, T.L.; Nielsen, P.M. Effects of Papain and a Microbial Enzyme on Meat Proteins and Beef Tenderness. J. Food Sci. 2002, 67, 2138–2142. [Google Scholar] [CrossRef]
- Christensen, M.; Tørngren, M.A.; Gunvig, A.; Rozlosnik, N.; Lametsch, R.; Karlsson, A.H.; Ertbjerg, P. Injection of marinade with actinidin increases tenderness of porcine M. biceps femoris and affects myofibrils and connective tissue. J. Sci. Food Agric. 2009, 89, 1607–1614. [Google Scholar] [CrossRef]
- Zhu, X.; Kaur, L.; Staincliffe, M.; Boland, M. Actinidin Pretreatment and Sous Vide Cooking of Beef Brisket: Effects on Meat Microstructure, Texture and in Vitro Protein Digestibility. Meat Sci. 2018, 145, 256–265. [Google Scholar] [CrossRef]
- Naqvi, Z.B.; Campbell, M.A.; Latif, S.; Thomson, P.C.; McGill, D.M.; Warner, R.D.; Friend, M.A. Improving tenderness and quality of M. biceps femoris from older cows through concentrate feeding, zingibain protease and sous vide cooking. Meat Sci. 2021, 180, 108563. [Google Scholar] [CrossRef]
- Pawar, V.D.; Mule, B.D.; Machewad, G.M. Effect of marination with ginger rhizome extract on properties of raw and cooked chewon. J. Muscle Foods 2007, 18, 349–369. [Google Scholar] [CrossRef]
- Naveena, B.M.; Mendiratta, S.K. The tenderization of bufalo meat using ginger extract. J. Muscle Foods 2005, 15, 235–244. [Google Scholar] [CrossRef]
- Moon, S.S. Effect of proteolytic enzymes and ginger extract on tenderization of m. pectoralis profundus from Holstein steer. Korean J. Food Sci. Anim. Resour. 2018, 38, 143. [Google Scholar]
- Bhaskar, N.; Sachindra, N.M.; Modi, V.K.; Sakhare, P.Z.; Mahendrakar, N.S. Preparation of proteolytic activity rich ginger powder and evaluation of its tenderizing effect on spent-hen muscles. J. Muscle Foods 2006, 17, 174–184. [Google Scholar] [CrossRef]
- Sullivan, G.A.; Calkins, C.R. Application of Exogenous Enzymes to Beef Muscle of High and Low-Connective Tissue. Meat Sci. 2010, 85, 730–734. [Google Scholar] [CrossRef]
- Christensen, L.; Ertbjerg, P.; Løje, H.; Risbo, J.; van den Berg, F.W.J.; Christensen, M. Relationship between Meat Toughness and Properties of Connective Tissue from Cows and Young Bulls Heat Treated at Low Temperatures for Prolonged Times. Meat Sci. 2013, 93, 787–795. [Google Scholar] [CrossRef]
- Ertbjerg, P.; Christiansen, L.S.; Pedersen, A.B.; Kristensen, L. The effect of temperature and time on activity of calpain and lysosomal enzymes and degradation of desmin in porcine longissimus muscle. In Proceedings of the 58th International Congress of Meat Science and Technology, Montreal, QC, Canada, 12–17 August 2012. [Google Scholar]
- BC Centre for Disease Control 2016-Guidelines for Restaurant Sous Vide Cooking Safety in British Columbia. Available online: http://www.bccdc.ca/search?k=svguidelines_finalforweb.pdf (accessed on 9 February 2021).
- Juneja, V.K.; Marmer, B.S. Thermal Inactivation of Clostridium Perfringens Vegetative Cells in Ground Beef and Turkey as Affected by Sodium Pyrophosphate. Food Microbiol. 1998, 15, 281–287. [Google Scholar] [CrossRef]
- ECFF (European Chilled Food Federation). Recommendations for the Production of Prepackaged Chilled Food; The European Chilled Food Federation: Helsinki, Finland, 2006; Available online: https://ecff.net/wp-content/uploads/2018/10/ECFF_Recommendations_2nd_ed_18_12_06.pdf (accessed on 20 January 2021).
- SVAC (Sous Vide Advisory Committee). Code of Practice for Sous Vide Catering Systems; SVAC: Tetbury, UK, 1991. [Google Scholar]
- AOAC. AOAC Official Methods of Analysis, 18th ed.; AOAC International: Rockville, MD, USA, 2005; ISBN 0935584870. [Google Scholar]
- Commission Internationale de L’Eclairage (CIE). Colorimetry, 2nd ed.; CIE: Viena, Austria, 1986; ISBN 3-900-734-00-3. [Google Scholar]
- Knispel, G. Factors affecting the process of color matching restorative materials to natural teeth. Quintessence Int. 1991, 22, 525–531. [Google Scholar]
- Bourne, M.C. Texture profile analysis. Food Tech. 1978, 32, 62. [Google Scholar]
- Dias, M.V.; Nilda de Fátima, F.S.; Borges, S.V.; de Sousa, M.M.; Nunes, C.A.; de Oliveira, I.R.N.; Medeiros, E.A.A. Use of Allyl Isothiocyanate and Carbon Nanotubes in an Antimicrobial Film to Package Shredded, Cooked Chicken Meat. Food Chem. 2013, 141, 3160–3166. [Google Scholar] [CrossRef] [PubMed]
- Ganhão, R.; Estévez, M.; Morcuende, D. Suitability of the TBA Method for Assessing Lipid Oxidation in a Meat System with Added Phenolic-Rich Materials. Food Chem. 2011, 126, 772–778. [Google Scholar] [CrossRef]
- Oliveira, T.L.C.; Junior, B.R.D.C.L.; Ramos, A.L.; Ramos, E.M.; Piccoli, R.H.; Cristianini, M. Phenolic carvacrol as a natural additive to improve the preservative effects of high pressure processing of low-sodium sliced vacuum-packed turkey breast ham. LWT-Food Sci. Technol. 2015, 64, 1297–1308. [Google Scholar] [CrossRef]
- Warner, R.D.; Kauffman, R.G.; Greaser, M.L. Muscle Protein Changes Post Mortem in Relation to Pork Quality Traits. Meat Sci. 1997, 45, 339–352. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Abel, T.; Boulaaba, A.; Lis, K.; Abdulmawjood, A.; Plötz, M.; Becker, A. Inactivation of Listeria monocytogenes in game meat applying sous vide cooking conditions. Meat Sci. 2020, 167, 108164. [Google Scholar] [CrossRef]
- Karyotis, D.; Skandamis, P.N.; Juneja, V.K. Thermal Inactivation of Listeria Monocytogenes and Salmonella Spp. In Sous-Vide Processed Marinated Chicken Breast. Food Res. Int. 2017, 100, 894–898. [Google Scholar] [CrossRef]
- Peck, M.W.; Goodburn, K.E.; Betts, R.P.; Stringer, S.C. Assessment of the potential for growth and neurotoxin formation by non-proteolytic Clostridium botulinum in short shelf-life commercial foods designed to be stored chilled. Trends Food Sci. Technol. 2008, 19, 207–216. [Google Scholar] [CrossRef]
- Hwang, S.-I.; Lee, E.-J.; Hong, G.-P. Effects of Temperature and Time on the Cookery Properties of Sous-Vide Processed Pork Loin. Food Sci. Anim. Resour. 2019, 39, 65–72. [Google Scholar] [CrossRef]
- Offer, G.; Restall, D.; Trinick, J. Water-holding in meat. Recent Adv. Chem. Meat 1984, 47, 71–86. [Google Scholar]
- Sałek, P.; Przybylski, W.; Jaworska, D.; Adamczak, L.; Zielińska, D.; Głuchowski, A. The Effects on the Quality of Poultry Meat of Supplementing Feed with Zinc-Methionine Complex. Acta Sci. Pol. Technol. Aliment. 2020, 19, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Aaslyng, M.D.; Bejerholm, C.; Ertbjerg, P.; Bertram, H.C.; Andersen, H.J. Cooking Loss and Juiciness of Pork in Relation to Raw Meat Quality and Cooking Procedure. Food Qual. Prefer. 2003, 14, 277–288. [Google Scholar] [CrossRef]
- Zielbauer, B.I.; Franz, J.; Viezens, B.; Vilgis, T.A. Physical Aspects of Meat Cooking: Time Dependent Thermal Protein Denaturation and Water Loss. Food Biophys. 2015, 11, 34–42. [Google Scholar] [CrossRef]
- Sánchez del Pulgar, J.; Gázquez, A.; Ruiz-Carrascal, J. Physico-Chemical, Textural and Structural Characteristics of Sous-Vide Cooked Pork Cheeks as Affected by Vacuum, Cooking Temperature, and Cooking Time. Meat Sci. 2012, 90, 828–835. [Google Scholar] [CrossRef]
- King, N.J.; Whyte, R. Does It Look Cooked? A Review of Factors That Influence Cooked Meat Color. J. Food Sci. 2006, 71, R31–R40. [Google Scholar] [CrossRef]
- Hunt, M.C.; Sorheim, O.; Slinde, E. Color and Heat Denaturation of Myoglobin Forms in Ground Beef. J. Food Sci. 1999, 64, 847–851. [Google Scholar] [CrossRef]
- Da Silva-Buzanello, R.A.; Schuch, A.F.; Gasparin, A.W.; Torquato, A.S.; Scremin, F.R.; Canan, C.; Soares, A.L. Quality Parameters of Chicken Breast Meat Affected by Carcass Scalding Conditions. Asian-Australas. J. Anim. Sci. 2019, 32, 1186–1194. [Google Scholar] [CrossRef]
- Haghighi, H.; Belmonte, A.M.; Masino, F.; Minelli, G.; Lo Fiego, D.P.; Pulvirenti, A. Effect of Time and Temperature on Physicochemical and Microbiological Properties of Sous Vide Chicken Breast Fillets. Appl. Sci. 2021, 11, 3189. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, B.; Oh, E.; Kim, Y.S.; Choi, Y.M. Combined effects of sous-vide cooking conditions on meat and sensory quality characteristics of chicken breast meat. Poult. Sci. 2020, 99, 3286–3291. [Google Scholar] [CrossRef]
- Langsrud, S.; Sørheim, O.; Skuland, S.E.; Almli, V.L.; Jensen, M.R.; Grøvlen, M.S.; Ueland, Ø.; Møretrø, T. Cooking Chicken at Home: Common or Recommended Approaches to Judge Doneness May Not Assure Sufficient Inactivation of Pathogens. PLoS ONE 2020, 15, e0230928. [Google Scholar] [CrossRef]
- Holownia, K.; Chinnan, M.; Reynolds, A.; Koehler, P. Evaluation of Induced Color Changes in Chicken Breast Meat during Simulation of Pink Color Defect. Poult. Sci. 2003, 82, 1049–1059. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Tatol, M. Colour difference∆ E-A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Tomasević, I.; Tomović, V.; Milovanović, B.; Lorenzo, J.; Đorđević, V.; Karabasil, N.; Đekić, I. Comparison of a computer vision system vs. traditional colorimeter for color evaluation of meat products with various physical properties. Meat Sci. 2019, 148, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Dransfield, E. Optimisation of Tenderisation, Ageing and Tenderness. Meat Sci. 1994, 36, 105–121. [Google Scholar] [CrossRef]
- Spanier, A.M.; McMillin, K.W.; Miller, J.A. Enzyme Activity Levels in Beef: Effect of Postmortem Aging and End-Point Cooking Temperature. J. Food Sci. 1990, 55, 318–322. [Google Scholar] [CrossRef]
- Forde, C.G.; van Kuijk, N.; Thaler, T.; de Graaf, C.; Martin, N. Texture and Savoury Taste Influences on Food Intake in a Realistic Hot Lunch Time Meal. Appetite 2013, 60, 180–186. [Google Scholar] [CrossRef]
- Estévez, M.; Ventanas, S.; Heinonen, M. Formation of Strecker Aldehydes between Protein Carbonyls–α-Aminoadipic and γ-Glutamic Semialdehydes–and Leucine and Isoleucine. Food Chem. 2011, 128, 1051–1057. [Google Scholar] [CrossRef]
- Akoğlu, I.; Bıyıklı, M.; Akoğlu, A.; Kurhan, Ş. Determination of the Quality and Shelf Life of Sous Vide Cooked Turkey Cutlet Stored at 4 and 12oC. Rev. Bras. Ciência Avícola 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Cropotova, J.; Mozuraityte, R.; Standal, I.B.; Rustad, T. The Influence of Cooking Parameters and Chilled Storage Time on Quality of Sous-Vide Atlantic Mackerel (Scomber Scombrus). J. Aquat. Food Prod. Technol. 2019, 28, 505–518. [Google Scholar] [CrossRef]
- Murphy, R.Y.; Marks, B.P. Effect of Meat Temperature on Proteins, Texture, and Cook Loss for Ground Chicken Breast Patties. Poult. Sci. 2000, 79, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.T.; Kauffman, R.G.; Kim, B.C.; Park, G.B. The Relationship of Sarcoplasmic and Myofibrillar Protein Solubility to Colour and Water-Holding Capacity in Porcine Longissimus Muscle. Meat Sci. 1999, 52, 291–297. [Google Scholar] [CrossRef]
| Group | Time at the Temperature of 50 °C (min) | Time at the Temperature of 60 °C (min) | Treatment Time Ratio 50 °C/60 °C | Total Treatment Time (min) |
|---|---|---|---|---|
| T1 | 0 | 120 | 0:1 | 120 |
| T2 | 40 | 80 | 1:2 | 120 |
| T3 | 60 | 60 | 1:1 | 120 |
| T4 | 0 | 180 | 0:1 | 180 |
| T5 | 60 | 120 | 1:2 | 180 |
| T6 | 90 | 90 | 1:1 | 180 |
| Total Treatment Time | 120 min | 180 min | ||||
|---|---|---|---|---|---|---|
| Treatment Time Ratio | 0:1 | 1:2 | 1:1 | 0:1 | 1:2 | 1:1 |
| Treatments | T1 | T2 | T3 | T4 | T5 | T6 |
| 130.26 ± 3.92 | 86.68 ± 5.09 | 67.57 ± 2.73 | 197.55 ± 7.73 | 136.17 ± 4.41 | 99.07 ± 3.70 | |
| 12.21 ± 1.06 | 9.31 ± 0.37 | 7.15 ± 0.68 | 17.34 ± 1.02 | 13.50 ± 0.71 | 10.06 ± 0.52 | |
| 4.01 ± 0.33 | 2.92 ± 0.19 | 2.18 ± 0.12 | 6.01 ± 0.31 | 4.47 ± 0.28 | 3.06 ± 0.16 | |
| Total Treatment Time | 120 min | 180 min | ||||
|---|---|---|---|---|---|---|
| Treatment Time Ratio | 0:1 | 1:2 | 1:1 | 0:1 | 1:2 | 1:1 |
| Treatments | T1 | T2 | T3 | T4 | T5 | T6 |
| Moisture content (%) | 71.21 ± 0.45 Ba | 72.09 ± 0.21 Ba | 71.94 ± 0.57 Ba | 68.9 ± 0.45 Aa | 69.73 ± 0.55 Aab | 70.12 ± 0.18 Ab |
| Cooking loss (%) | 13.43 ± 0.20 Ab | 11.82 ± 0.49 Aa | 11.61 ± 0.40 Aa | 16.35 ± 0.92 Bb | 14.74 ± 0.39 Ba | 12.92 ± 0.86 Aa |
| L* | 80.00 ± 0.24 Ab | 78.79 ± 0.30 Aa | 78.36 ± 0.23 Aa | 80.83 ± 0.05 Bc | 78.96 ± 0.22 Ab | 78.38 ± 0.21 Aa |
| a* | 1.79 ± 0.30 Aa | 2.10 ± 0.17 Aa | 2.22 ± 0.17 Aa | 1.36 ± 0.2 Aa | 1.94 ± 0.18 Ab | 2.49 ± 0.22 Ac |
| b* | 9.55 ± 0.18 Aa | 11.55 ± 0.39 Ab | 13.10 ± 0.13 Ac | 9.11 ± 0.34 Aa | 12.59 ± 0.39 Ab | 12.87 ± 0.65 Ab |
| ΔE | 29.34 ± 0.26 Ab | 28.64 ± 0.28 Aa | 28.69 ± 0.18 Aa | 30.06 ± 0.03 Bb | 29.08 ± 0.26 Aa | 28.65 ± 0.23 Aa |
| Total Color Difference (ΔE) | ||||||
|---|---|---|---|---|---|---|
| Treatments | T1 | T2 | T3 | T4 | T5 | T6 |
| T1 | - | |||||
| T2 | 2.36 (Cat. III) | - | ||||
| T3 | 3.94 (Cat. IV) | 1.61 (Cat. II) | - | |||
| T4 | 1.03 (Cat. II) | 3.27 (Cat. III) | 4.77 (Cat. IV) | - | ||
| T5 | 3.22 (Cat. III) | 1.07 (Cat. II) | 0.84 (Cat. I) | 3.99 (Cat. IV) | - | |
| T6 | 3.76 (Cat. IV) | 1.44 (Cat. II) | 0.36 (Cat. I) | 4.63 (Cat. IV) | 0.85 (Cat. I) | - |
| Total Treatment Time | 120 min | 180 min | ||||
|---|---|---|---|---|---|---|
| Treatment Time Ratio | 0:1 | 1:2 | 1:1 | 0:1 | 1:2 | 1:1 |
| Treatments | T1 | T2 | T3 | T4 | T5 | T6 |
| Shear force (N) | 26.17 ± 2.37 Aa | 22.65 ± 1.24 Ba | 22.37 ± 1.17 Ba | 24.38 ± 2.05 Ab | 19.70 ± 0.58 Aa | 19.28 ± 0.65 Aa |
| Hardness (N) | 49.78 ± 1.05 Ab | 39.84 ± 3.44 Aa | 37.79 ± 4.16 Aa | 46.45 ± 3.39 Aa | 44.55 ± 4.70 Aa | 41.08 ± 3.91 Aa |
| Cohesiveness (-) | 0.23 ± 0.01 Aab | 0.19 ± 0.01 Aa | 0.25 ± 0.03 Ab | 0.26 ± 0.02 Aa | 0.27 ± 0.02 Ba | 0.24 ± 0.02 Aa |
| Springiness (mm) | 1.47 ± 0.11 Aa | 1.47 ± 0.09 Aa | 1.51 ± 0.04 Aa | 1.43 ± 0.08 Aa | 1.52 ± 0.15 Aa | 1.50 ± 0.05 Aa |
| Gumminess (N) | 11.30 ± 0.30 Ab | 7.38 ± 0.64 Aa | 9.34 ± 0.10 Aa | 12.11 ± 0.32 Ab | 11.89 ± 0.23 Bb | 9.70 ± 0.11 Aa |
| Chewiness (N * mm) | 16.58 ± 1.45 Ab | 10.86 ± 1.65 Aa | 14.05 ± 0.33 Aab | 17.38 ± 1.37 Aab | 18.07 ± 1.88 Bb | 14.52 ± 0.64 Aa |
| Total Treatment Time | 120 min | 180 min | ||||
|---|---|---|---|---|---|---|
| Treatment Time Ratio | 0:1 | 1:2 | 1:1 | 0:1 | 1:2 | 1:1 |
| Treatments | T1 | T2 | T3 | T4 | T5 | T6 |
| TBARS (mg MDA/kg) | 0.36 ± 0.01 Ac | 0.29 ± 0.01 Ab | 0.23 ± 0.02 Aa | 0.52 ± 0.03 Bb | 0.44 ± 0.02 Ba | 0.39 ± 0.03 Ba |
| Sarcoplasmic protein solubility (g/100 g meat) | 2.81 ± 0.16 Aa | 3.21 ± 0.06 Ab | 3.90 ± 0.09 Ac | 2.97 ± 0.07 Aa | 3.19 ± 0.07 Aa | 3.51 ± 0.14 Ab |
| Myofibrillar protein solubility (g/100 g meat) | 4.20 ± 0.06 Ba | 4.26 ± 0.05 Ba | 4.22 ± 0.11 Aa | 3.19 ± 0.29 Aa | 3.71 ± 0.07 Ab | 3.94 ± 0.18 Ab |
| Total protein solubility (g/100 g meat) | 7.02 ± 0.13 Aa | 7.47 ± 0.10 Bb | 8.12 ± 0.15 Bc | 6.15 ± 0.31 Aa | 6.90 ± 0.13 Ab | 7.45 ± 0.13 Ac |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasani, E.; Csehi, B.; Darnay, L.; Ladányi, M.; Dalmadi, I.; Kenesei, G. Effect of Combination of Time and Temperature on Quality Characteristics of Sous Vide Chicken Breast. Foods 2022, 11, 521. https://doi.org/10.3390/foods11040521
Hasani E, Csehi B, Darnay L, Ladányi M, Dalmadi I, Kenesei G. Effect of Combination of Time and Temperature on Quality Characteristics of Sous Vide Chicken Breast. Foods. 2022; 11(4):521. https://doi.org/10.3390/foods11040521
Chicago/Turabian StyleHasani, Endrit, Barbara Csehi, Lívia Darnay, Márta Ladányi, István Dalmadi, and György Kenesei. 2022. "Effect of Combination of Time and Temperature on Quality Characteristics of Sous Vide Chicken Breast" Foods 11, no. 4: 521. https://doi.org/10.3390/foods11040521