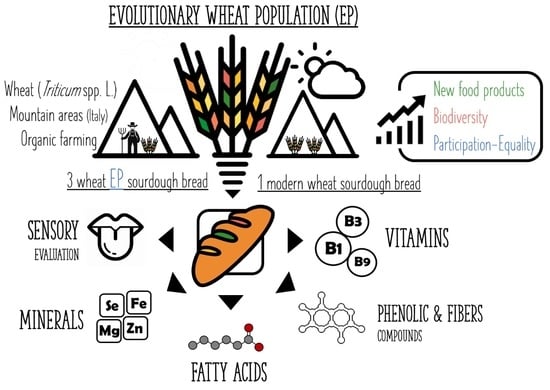
Evolutionary Wheat Populations in High-Quality Breadmaking as a Tool to Preserve Agri-Food Biodiversity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Cereal Grain Milling and Bread Formulation
2.3.1. Technological Quality Analysis of the Wheat Flours
2.3.2. Flour Preparation
2.3.3. Bread Formulation
2.4. Protein, Lipids, Dietary Fibre Components and Carbohydrates of Breads
2.5. Determination of Magnesium (Mg), Zinc (Zn), Iron (Fe), Selenium (Se) Content of Flours and Breads
2.6. Sample Extraction for Soluble and Insoluble Phenolic Compounds of Flours and Breads
2.6.1. Soluble and Insoluble Total Phenolic Content (TPC)
2.6.2. Soluble and Insoluble Phenolic Acids Profile Using UHPLC-MS/MS
2.7. Determination of Thiamine, Nicotinic Acid and Nicotinamide, and Folic Acid Content
2.8. Acceptability and Check-All-That-Apply (CATA) Analysis of Formulated Breads
2.9. Statistical Analysis
3. Results and Discussion
3.1. Milling and Technological Quality of Wheat
3.2. Lipid Content and Fatty Acids Profile of Breads
3.3. Total Dietary Fibre (TDF) and DF Classes of Flours and Breads
3.4. Selected Micronutrients Content of Flours and Breads
3.5. Phenolic Compounds from Flours to Breads
3.6. Check-All-That-Apply (CATA) Analysis of Breads
4. Conclusions
5. Study Limitations and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Statement
Abbreviations
| BB | bread produced using BIO2 EP |
| BBo | bread produced using cv. Bologna; |
| BG | bread produced using Grossi EP; |
| BI | bread produced using ICARDA EP; |
| CATA | check-all-that-apply analysis; |
| CA | correspondence analysis; |
| EP | evolutionary wheat population; |
| ER | extraction rate; |
| FA | fatty acids; |
| FB | BIO2 EP Type 1 flour; |
| FBo | cv. Bologna Type 1 flour; |
| FG | Grossi EP Type 1 flour; |
| FI | ICARDA EP Type 1 flour; |
| GAE | gallic acid equivalents; |
| GC-MS | gas chromatography coupled to mass spectrometry; |
| HMWIDF | high molecular weight insoluble dietary fibre; |
| HMWSDF | high molecular weight soluble dietary fibre; |
| ICP-MS | inductively coupled plasma with mass spectrometer; |
| LMWSDF | low molecular weight soluble dietary fibre; |
| MUFA | monounsaturated fatty acids; |
| MRM | multiple reaction monitoring; |
| NIR | near infrared; |
| ANOVA | analysis of variance; |
| PUFA | polyunsaturated fatty acids; |
| SFA | saturated fatty acids; |
| TDF | total dietary fibre, |
| TPC | total phenolic content; |
| UHPLC-MS/MS | ultra-high-performance liquid chromatography coupled to tandem mass spectrometry; |
| UPLC-ESI-LTQ/MS | ultra-performance liquid chromatograph electrospray ionization coupled to linear trap quadrupole mass spectrometer. |
References
- Malézieux, E.; Crozat, Y.; Dupraz, C.; Laurans, M.; Makowski, D.; Ozier-Lafontaine, H.; Rapidel, B.; de Tourdonnet, S.; Val-antin-Morison, M. Mixing plant species in cropping systems: Concepts, tools and models: A review. Agron. Sustain. Dev. 2009, 29, 43–62. [Google Scholar] [CrossRef] [Green Version]
- Bowles, T.M.; Mooshammer, M.; Socolar, Y.; Calderón, F.; Cavigelli, M.A.; Culman, S.W.; Deen, W.; Drury, C.F.; Garcia, A.G.Y.; Gaudin, A.C.M.; et al. Long-term evidence shows that crop-rotation diversification increases agricultural resilience to adverse growing conditions in North America. One Earth 2020, 2, 284–293. [Google Scholar] [CrossRef]
- Weiner, J.; Andersen, S.B.; Wille, W.K.M.; Griepentrog, H.W.; Olsen, J.M. Evolutionary Agroecology: The potential for cooperative, high density, weed-suppressing cereals. Evol. Appl. 2010, 3, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Lammer, D.; Lyon, S.; Carter, B.; Jones, S.S. Breeding for organic and low-input farming systems: An evolutionary–participatory breeding method for inbred cereal grains. Renew. Agric. Food Syst. 2005, 20, 48–55. [Google Scholar] [CrossRef]
- Suneson, C.A. An Evolutionary Plant Breeding Method 1. J. Agron. 1956, 48, 188–191. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S. Participatory plant breeding: Who did it, who does it and where? Exp. Agric. 2019, 56, 1–11. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S.; Maatougui, M.; Michael, M.; Slash, M.; Haghparast, R.; Rahmanian, M.; Taheri, A.; Al-Yassin, A.; Benbelkacem, A.; et al. Plant breeding and climate changes. J. Agric. Sci. 2010, 148, 627–637. [Google Scholar] [CrossRef]
- Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on Organic Production and Labelling of Organic Products and Repealing Council Regulation (EC) No 834/2007. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX:32018R0848 (accessed on 7 November 2021).
- Batur, F.; Bocci, R.; Bartha, B. Marketing Farmers’ Varieties in Europe: Encouraging Pathways with Missing Links for the Recognition and Support of Farmer Seed Systems. Agronomy 2021, 11, 2159. [Google Scholar] [CrossRef]
- Migliorini, P.; Spagnolo, S.; Torri, L.; Arnoulet, M.; Lazzerini, G.; Ceccarelli, S. Agronomic and quality characteristics of old, modern and mixture wheat varieties and landraces for organic bread chain in diverse environments of northern Italy. Eur. J Agron. 2016, 79, 131–141. [Google Scholar] [CrossRef]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol. 2009, 26, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Serrazanetti, D.I.; Tassoni, A.; Baldissarri, M.; Gianotti, A. Improving the functional and sensorial profile of cereal-based fermented foods by selecting Lactobacillus plantarum strains via a metabolomics approach. Food Res. Int. 2016, 89, 1095–1105. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Hersleth, M.; Berggren, R.; Westad, F.; Martens, M. Perception of bread: A comparison of consumers and trained assessors. J. Food Sci. 2005, 70, S95–S101. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Bartkiene, E.; Florença, S.G.; Djekić, I.; Bizjak, M.Č.; Tarcea, M.; Leal, M.; Ferreira, V.; Rumbak, I.; Orfanos, P.; et al. Environmental Issues as Drivers for Food Choice: Study from a Multinational Framework. Sustainability 2021, 13, 2869. [Google Scholar] [CrossRef]
- Valentin, D.; Chollet, S.; Lelièvre, M.; Abdi, H. Quick and dirty but still pretty good: A review of new descriptive methods in food science. Int. J. Food Sci. Technol. 2012, 47, 1563–1578. [Google Scholar] [CrossRef]
- Varela, P.; Ares, G. Sensory profiling, the blurred line between sensory and consumer science. A review of novel methods for product characterization. Food Res. Int. 2012, 48, 893–908. [Google Scholar] [CrossRef]
- Ares, G.; Antúnez, L.; Giménez, A.; Roigard, C.M.; Pineau, B.; Hunter, D.C.; Jaeger, S.R. Further investigations into the reproducibility of check-all-that-apply (CATA) questions for sensory product characterization elicited by consumers. Food Qual. Prefer. 2014, 36, 111–121. [Google Scholar] [CrossRef]
- Nguyen, Q.C.; Wahlgren, M.B.; Almli, V.L.; Varela, P. Understanding the role of dynamic texture perception in consumers’ expectations of satiety and satiation. A case study on barley bread. Food Qual. Prefer. 2017, 62, 218–226. [Google Scholar] [CrossRef]
- Cunha, L.M.; Fonseca, S.C.; Lima, R.C.; Loureiro, J.; Pinto, A.S.; Vaz Patto, M.C.; Brites, C. Consumer-driven improvement of maize bread formulations with legume fortification. Foods 2019, 8, 235. [Google Scholar] [CrossRef] [Green Version]
- Boukid, F.; Gentilucci, V.; Vittadini, E.; De Montis, A.; Rosta, R.; Bosi, S.; Dinelli, G.; Carini, E. Rediscovering bread quality of “old” Italian wheat (Triticum aestivum L. ssp. aestivum.) through an integrated approach: Physicochemical evaluation and consumers’ perception. LWT 2020, 122, 109043. [Google Scholar] [CrossRef]
- Piga, A.; Conte, P.; Fois, S.; Catzeddu, P.; Del Caro, A.; Sanguinetti, A.M.; Fadda, C. Technological, Nutritional and Sensory Properties of an Innovative Gluten-Free Double-Layered Flat Bread Enriched with Amaranth Flour. Foods 2021, 10, 920. [Google Scholar] [CrossRef]
- Ares, G.; Tárrega, A.; Izquierdo, L.; Jaeger, S.R. Investigation of the number of consumers necessary to obtain stable sample and descriptor configurations from check-all-that-apply (CATA) questions. Food Qual. Prefer. 2014, 31, 135–141. [Google Scholar] [CrossRef]
- SR EN ISO 27971; Cereals and Cereal Products—Common Wheat (Triticum aestivum L.). Determination of Alveograph Properties of Dough at Constant Hydration from Commercial or Test Flours and Test Milling Methodology. International Organization for Standardization: Geneva, Switzerland, 2008.
- AACC International. Approved Methods of Analysis, 11th ed.; Method 08-12.01; Ash in Farina and Semolina; AACC International: St. Paul, MN, USA, 1999; Available online: https://www.cerealsgrains.org/resources/Methods/Pages/08TotalAsh.aspx (accessed on 7 November 2021).
- Decreto Presidente della Repubblica 187. Regolamento per la Revisione della Normativa sulla Produzione e Commercializzazione di Sfarinati e Paste Alimentari. 2001. Available online: https://www.politicheagricole.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/2772 (accessed on 7 November 2021).
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists 402, 16th ed.; Science and Education: Washington, DC, USA, 1995. [Google Scholar]
- Dall’Asta, C.; Cirlini, M.; Morini, E.; Rinaldi, M.; Ganino, T.; Chiavaro, E. Effect of chestnut flour supplementation on physico-chemical properties and volatiles in bread making. LWT 2013, 53, 233–239. [Google Scholar] [CrossRef]
- ISO/IEC 17025:2005; General Requirements for the Competence of Testing and Calibration Laboratories, 2nd ed. International Organization for Standardization: Geneva, Switzerland, 2005.
- UNI EN 13805:2014; Foodstuffs—Determination of Trace Elements—Pressure Digestion. European Committee for Standardization: Brussels, Belgium, 2014.
- Zaupa, M.; Calani, L.; Del Rio, D.; Brighenti, F.; Pellegrini, N. Characterization of total antioxidant capacity and (poly)phenolic compounds of differently pigmented rice varieties and their changes during domestic cooking. Food Chem. 2015, 187, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Spaggiari, M.; Calani, L.; Folloni, S.; Ranieri, R.; Dall’Asta, C.; Galaverna, G. The impact of processing on the phenolic acids, free betaine and choline in Triticum spp. L. whole grains and milling by-products. Food Chem. 2020, 311, 125940. [Google Scholar] [CrossRef]
- Leporati, A.; Catellani, D.; Suman, M.; Andreoli, R.; Manini, P.; Niessen, W.M.A. Application of a liquid chromatography tandem mass spectrometry method to the analysis of water-soluble vitamins in Italian pasta. Anal. Chim. Acta 2005, 531, 87–95. [Google Scholar] [CrossRef]
- Mefleh, M.; Conte, P.; Fadda, C.; Giunta, F.; Piga, A.; Hassoun, G.; Motzo, R. From ancient to old and modern durum wheat varieties: Interaction among cultivar traits, management, and technological quality. J. Sci. Food Agric. 2019, 99, 2059–2067. [Google Scholar] [CrossRef]
- Oury, F.X.; Berard, P.; Brancourt-Hulmel, M.; Heumez, E.; Pluchard, P.; Rousset, M.; Doussinault, G.; Rolland, B.; Trottet, M.; Giraud, A.; et al. Yield and Grain Protein Concentration in Bread Wheat: A Review and a Study of Multi-Annual Data from a French Breeding Program [Triticum aestivum L.]. J. Genet. Breed. 2003, 57, 59–68. Available online: https://agris.fao.org/agris-search/search.do?recordID=IT2005601351 (accessed on 7 November 2021).
- Huen, J.; Börsmann, J.; Matullat, I.; Böhm, L.; Stukenborg, F.; Heitmann, M.; Zannini, E.; Arendt, E.K. Wheat flour quality evaluation from the baker’s perspective: Comparative assessment of 18 analytical methods. Eur. Food Res. Technol. 2018, 244, 535–545. [Google Scholar] [CrossRef]
- SIS (Società Italiana Sementi). Available online: http://www.sisonweb.com/images/allProdotti/Bologna%202015.pdf (accessed on 13 November 2021).
- Heiniö, R.L.; Noort, M.W.J.; Katina, K.; Alam, S.A.; Sozer, N.; de Kock, H.L.; Hersleth, M.; Poutanen, K. Sensory characteristics of wholegrain and bran-rich cereal foods—A review. Trends Food Sci. Technol. 2016, 47, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Tavoletti, S.; Foligni, R.; Mozzon, M.; Pasquini, M. Comparison between fatty acid profiles of old and modern varieties of T. turgidum and T. aestivum: A case study in central Italy. J. Cereal Sci. 2018, 82, 198–205. [Google Scholar] [CrossRef]
- Melis, S.; Delcour, J.A. Impact of wheat endogenous lipids on the quality of fresh bread: Key terms, concepts, and underlying mechanisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3715–3754. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2016, 8, 1461. [Google Scholar]
- Raninen, K.; Lappi, J.; Mykkänen, H.; Poutanen, K. Dietary fiber type reflects physiological functionality: Comparison of grain fiber, inulin, and polydextrose. Nutr. Rev. 2011, 69, 9–21. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- European Commission. EU Register on Nutrition and Health Claims Made on Foods. 2006, OJEU. Available online: https://ec.europa.eu/food/safety/labelling_nutrition/claims/register/public/?event=register.home (accessed on 7 November 2021).
- Amaral, O.; Guerreiro, C.S.; Gomes, A.; Cravo, M. Resistant starch production in wheat bread: Effect of ingredients, baking conditions and storage. Eur. Food Res. Technol. 2016, 242, 1747–1753. [Google Scholar] [CrossRef]
- Djurle, S.; Andersson, A.A.M.; Andersson, R. Effects of baking on dietary fibre, with emphasis on β-glucan and resistant starch, in barley breads. J. Cereal Sci. 2018, 79, 449–455. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Z.; Liu, N.; Zhou, P.; Bao, Q.; Wang, X. Effect of wheat bran dietary fibre on the rheological properties of dough during fermentation and Chinese steamed bread quality. Int. J. Food Sci. Technol. 2021, 56, 1623–1630. [Google Scholar] [CrossRef]
- AESAN/BEDCA. Base de Datos Española de Composición de Alimentos. 2018, V 1.0. Available online: https://www.bedca.net/bdpub/ (accessed on 7 November 2021).
- Gnagnarella, P.; Parpinel, M.; Salvini, S.; Franceschi, S.; Palli, D.; Boyle, P. The update of the Italian Food Composition Database. J. Food Comp. Anal. 2004, 17, 509–522. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture (USDA), A. R. S. USDA National Nutrient Database for Standard Reference, Release 27. 2014, In Database. Available online: https://data.nal.usda.gov/dataset/composition-foods-raw-processed-prepared-usda-national-nutrient-database-standard-reference-release-27 (accessed on 7 November 2021).
- Martins, Z.E.; Pinto, E.; Almeida, A.A.; Pinho, O.; Ferreira, I.M.P.L.V.O. Fibre fortification of wheat bread: Impact on mineral composition and bioaccessibility. Food Funct. 2017, 8, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.J.; Fairweather-Tait, S.J.; Broadley, M.R.; Dickinson, S.J.; Foot, I.; Knott, P.; McGrath, S.P.; Mowat, H.; Norman, K.; Scott, P.R.; et al. Selenium concentration and speciation in biofortified flour and bread: Retention of selenium during grain biofortification, processing and production of Se-enriched food. Food Chem. 2011, 126, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Mihhalevski, A.; Nisamedtinov, I.; Hälvin, K.; Ošeka, A.; Paalme, T. Stability of B-complex vitamins and dietary fiber during rye sourdough bread production. J. Cereal Sci. 2013, 57, 30–38. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel insights on the functional/nutritional features of the sourdough fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef]
- Batifoulier, F.; Verny, M.A.; Chanliaud, E.; Rémésy, C.; Demigné, C. Variability of B vitamin concentrations in wheat grain, milling fractions and bread products. Eur. J. Agron. 2006, 25, 163–169. [Google Scholar] [CrossRef]
- Brandolini, A.; Castoldi, P.; Plizzari, L.; Hidalgo, A. Phenolic acids composition, total polyphenols content and antioxidant activity of Triticum monococcum, Triticum turgidum and Triticum aestivum: A two-years evaluation. J. Cereal Sci. 2013, 58, 123–131. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Rabalski, I. Effect of baking on free and bound phenolic acids in wholegrain bakery products. J. Cereal Sci. 2013, 57, 312–318. [Google Scholar] [CrossRef]
- Anson, N.M.; Selinheimo, E.; Havenaar, R.; Aura, A.-M.; Mattila, I.; Lehtinen, P.; Bast, A.; Poutanen, K.; Haenen, G. Bioprocessing of wheat bran improves in vitro bioaccessibility and colonic metabolism of phenolic compounds. J. Agric. Food Chem. 2009, 57, 6148–6155. [Google Scholar] [CrossRef]
- Tian, W.; Chen, G.; Tilley, M.; Li, Y. Changes in phenolic profiles and antioxidant activities during the whole wheat bread-making process. Food Chem. 2021, 345, 128851. [Google Scholar] [CrossRef]
- Michalska, A.; Amigo-Benavent, M.; Zielinski, H.; del Castillo, M.D. Effect of bread making on formation of Maillard reaction products contributing to the overall antioxidant activity of rye bread. J. Cereal Sci. 2008, 48, 123–132. [Google Scholar] [CrossRef]
- Fares, C.; Menga, V.; Codianni, P.; Russo, M.; Perrone, D.; Suriano, S.; Savino, M.; Rascio, A. Phenolic acids variability and grain quality of organically and conventionally fertilised old wheats under a warm climate. J. Sci. Food Agric. 2019, 99, 4615–4623. [Google Scholar] [CrossRef] [PubMed]
- Katina, K.; Heiniö, R.-L.; Autio, K.; Poutanen, K. Optimization of sourdough process for improved sensory profile and texture of wheat bread. LWT-Food Sci. Technol. 2006, 39, 1189–1202. [Google Scholar] [CrossRef]



| Wheat | Test Weight (kg/hL) | Thousand Kernel Weight (g) | Protein Content (% d.m.) | Alveograph | |||
|---|---|---|---|---|---|---|---|
| W (10−4 J) | P (mm H2O) | L (mm) | P/L | ||||
| Bio2 EP | 74 a | 44 b | 16.82 b | 130.5 b | 67.5 d | 134.0 b | 0.5 a |
| ICARDA EP | 78 a | 45 b | 16.39 b | 152.5 c | 59.0 c | 129.5 b | 0.5 a |
| Grossi EP | 77 a | 47 c | 16.93 b | 106.5 a | 55.0 b | 108.5 a | 0.5 a |
| Bologna | 79 a | 32 a | 13.27 a | 288.0 d | 48.5 a | 98.5 a | 0.5 a |
| BB | BI | BG | BBo | |
|---|---|---|---|---|
| Energetic value (kJ) * | 1005.0 | 1058.1 | 1041.1 | 961.6 |
| Energetic value (kcal) * | 240.2 | 252.9 | 248.8 | 229.8 |
| Carbohydrates (g/100 g) | 48.3 a | 49.7 a | 47.7 a | 46.2 a |
| Total dietary fibre (g/100 g) | 4.55 a | 4.22 a | 4.64 a | 5.18 b |
| Lipids (g/100 g) | 0.83 a | 1.0 b | 1.20 c | 1.22 c |
| SFA (%) | 31.8 a | 32.2 a | 31.7 a | 31.0 a |
| MUFA (%) | 42.9 a | 45.2 b | 45.2 b | 42.7 a |
| PUFA (%) | 25.3 c | 22.6 a | 23.0 b | 26.3 d |
| Ω-6/Ω-9 | 0.53 b | 0.45 a | 0.45 a | 0.55 b |
| Proteins (g/100 g) | 12.4 a | 11.3 a | 12.1 a | 11.3 a |
| Mg (mg/100 g) | 24.5 a | 22.1 a | 24.1 a | 31.6 b |
| Zn (mg/100 g) | 0.85 b | 0.75 a | 0.82 a | 0.82 a |
| Fe (mg/100 g) | 1.37 c | 0.86 a | 1.09 b | 1.38 c |
| Se (µg/100 g) | 8.07 a | 8.11 a | 8.95 b | 8.77 b |
| Thiamine (mg/100 g) | 0.24 b | 0.18 a | 0.20 a | 0.43 c |
| Nicotinic acid (mg/100 g) | <LOQ | <LOQ | <LOQ | <LOQ |
| Folic acid (µg/100 g) | <LOQ | <LOQ | <LOQ | <LOQ |
| Nicotinamide (mg/100 g) | 1.77 b | 1.75 ab | 1.62 a | 2.18 c |
| FB | FI | FG | FBo | |
|---|---|---|---|---|
| Mg (mg/100 g) * | 29.2 a | 26.1 a | 28.5 a | 44.2 b |
| Zn (mg/100 g) * | 1.13 b | 0.97 a | 1.08 a | 1.17 b |
| Fe (mg/100 g) * | 1.80 c | 1.02 a | 1.29 b | 1.85 c |
| Se (µg/100 g) ** | 2.66 a | 2.40 a | 3.39 b | 3.96 b |
| Thiamine (mg/100 g) * | 0.29 b | 0.22 a | 0.33 bc | 0.36 c |
| Nicotinic acid (mg/100 g) * | <LOQ | <LOQ | <LOQ | <LOQ |
| Nicotinamide (mg/100 g) * | 0.43 a | <LOQ | 0.51 ab | 0.56 b |
| Folic acid (µg/100 g) ** | 21.8 b | <LOQ a | <LOQ a | <LOQ a |
| Sample Flours | TPC | 4-HB | p-C | Caff | t-Fer | c-Fer | Sin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Free | Bound | Free | Bound | Free | Bound | Free | Bound | Free | Bound | Free | Bound | ||
| mg GAE/Kg d.m. | mg/Kg d. m. | ||||||||||||
| FB | 256.22 a | 895.95 a | 0.07 a | <LOQ | <LOQ | 0.11 a | 0.21 b | 0.25 a | 1.53 b | 2.48 b | 0.59 a | 0.90 b | 1.25 b |
| FI | 217.70 a | 838.51 a | 0.09 a | <LOQ | 0.07 a | 0.12 a | 0.17 a | 0.25 a | 1.56 b | 2.21 a | 1.10 b | 0.80 a | 0.99 a |
| FG | 221.76 a | 912.84 a | <LOQ | <LOQ | 0.08 a | 0.12 a | 0.23 b | 0.32 b | 1.33 a | 3.25 c | 1.05 b | 0.96 b | 1.32 b |
| FBo | 390.49 b | 855.41 a | <LOQ | <LOQ | <LOQ | 0.18 b | 0.37 c | 0.52 c | 1.73 b | 2.66 ab | 0.91 b | 0.82 a | 1.17 b |
| Breads | |||||||||||||
| BB | 343.81 a | 217.97 a | <LOQ | 0.51 a | <LOQ | 1.47 b | <LOQ | 0.37 a | 1.32 a | 44.25 a | 21.47 a | <LOQ | 4.07 b |
| BI | 480.93 c | 182.89 a | <LOQ | 0.45 a | <LOQ | 1.21 a | <LOQ | 0.29 a | 1.86 a | 42.16 a | 15.96 a | 0.34 a | 3.73 a |
| BG | 414.83 b | 186.81 a | <LOQ | 0.73 b | 0.11 a | 1.79 c | <LOQ | 0.32 a | 2.29 ab | 47.23 a | 26.76 a | 0.38 a | 6.12 c |
| BBo | 490.29 c | 355.86 b | 0.21 a | 0.94 c | 0.11 a | 2.31 c | <LOQ | 0.63 b | 2.41 b | 73.64 b | 25.52 a | <LOQ | 6.19 c |
| Bread | Texture | Colour | Appearance | Aroma | Taste | Overall Acceptability | ||
|---|---|---|---|---|---|---|---|---|
| Crust | Crumb | Crust | Crumb | |||||
| BI | 6.95 b | 7.05 a | 6.81 a | 6.78 ab | 7.05 a | 6.51 a | 6.69 a | 7.02 b |
| BB | 6.71 b | 6.92 a | 6.78 a | 6.97 b | 7.00 a | 6.46 a | 6.42 a | 6.73 ab |
| BG | 6.85 b | 6.78 a | 6.88 a | 7.10 b | 7.15 a | 6.27 a | 6.15 a | 6.75 ab |
| BBo | 6.08 a | 6.41 a | 6.83 a | 6.39 a | 6.59 a | 6.24 a | 6.08 a | 6.36 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spaggiari, M.; Marchini, M.; Calani, L.; Dodi, R.; Di Pede, G.; Dall’Asta, M.; Scazzina, F.; Barbieri, A.; Righetti, L.; Folloni, S.; et al. Evolutionary Wheat Populations in High-Quality Breadmaking as a Tool to Preserve Agri-Food Biodiversity. Foods 2022, 11, 495. https://doi.org/10.3390/foods11040495
Spaggiari M, Marchini M, Calani L, Dodi R, Di Pede G, Dall’Asta M, Scazzina F, Barbieri A, Righetti L, Folloni S, et al. Evolutionary Wheat Populations in High-Quality Breadmaking as a Tool to Preserve Agri-Food Biodiversity. Foods. 2022; 11(4):495. https://doi.org/10.3390/foods11040495
Chicago/Turabian StyleSpaggiari, Marco, Mia Marchini, Luca Calani, Rossella Dodi, Giuseppe Di Pede, Margherita Dall’Asta, Francesca Scazzina, Andrea Barbieri, Laura Righetti, Silvia Folloni, and et al. 2022. "Evolutionary Wheat Populations in High-Quality Breadmaking as a Tool to Preserve Agri-Food Biodiversity" Foods 11, no. 4: 495. https://doi.org/10.3390/foods11040495