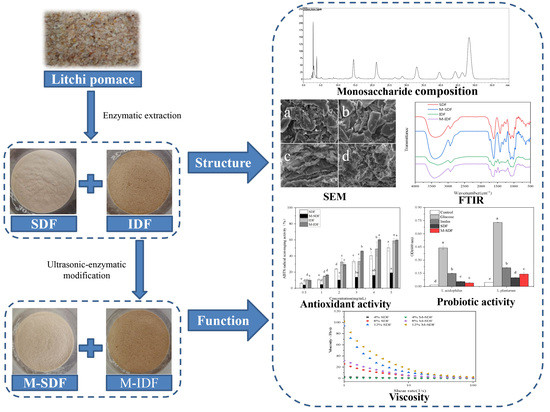
Comparison the Structural, Physicochemical, and Prebiotic Properties of Litchi Pomace Dietary Fibers before and after Modification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Dietary Fiber from Litchi Pomace
2.3. Ultrasonic Enzymatic Modification of IDF from Litchi Pomace
2.4. Monosaccharide Composition
2.5. Scanning Electron Microscopy (SEM)
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.7. Hydration Properties
2.8. Rheological Property
2.9. The Radical Scavenging Activity
2.10. In Vitro Probiotic Activity
2.11. Statistical Analysis
3. Results and Discussion
3.1. Structural Characterization
3.1.1. Monosaccharide Composition Analysis
3.1.2. SEM Analysis
3.1.3. FTIR Analysis
3.2. Physicochemical Properties
3.2.1. Hydration Properties
3.2.2. Rheological Properties
3.3. Functional Properties
3.3.1. The Radical Scavenging Activity
3.3.2. In Vitro Probiotic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pareek, S. Nutritional and Biochemical Composition of Lychee (Litchi chinensis Sonn.) Cultivars. In Nutritional Composition of Fruit Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Wang, Y.; Song, L.; Liu, H.; Lichter, A.; Kerdchoechuen, O.; Joyce, D.C.; Shi, J. Postharvest characteristics and handling of litchi fruit—An overview. Aust. J. Exp. Agric. 2006, 46, 1541–1556. [Google Scholar] [CrossRef]
- Zhu, X.-R.; Wang, H.; Sun, J.; Yang, B.; Duan, X.-W.; Jiang, Y.-M. Pericarp and seed of litchi and longan fruits: Constituent, extraction, bioactive activity, and potential utilization. J. Zhejiang Univ. Sci. B 2019, 20, 503–512. [Google Scholar] [CrossRef]
- Pop, C.; Suharoschi, R.; Pop, O. Dietary Fiber and Prebiotic Compounds in Fruits and Vegetables Food Waste. Sustainability 2021, 13, 7219. [Google Scholar] [CrossRef]
- Emanuele, S.; Lauricella, M.; Calvaruso, G.; D’Anneo, A.; Giuliano, M. Litchi chinensis as a Functional Food and a Source of Antitumor Compounds: An Overview and a Description of Biochemical Pathways. Nutrients 2017, 9, 992. [Google Scholar] [CrossRef] [Green Version]
- Su, D.; Zhang, R.; Zhang, C.; Huang, F.; Xiao, J.; Deng, Y.; Wei, Z.; Zhang, Y.; Chi, J.; Zhang, M. Phenolic-rich lychee (Litchi chinensis Sonn.) pulp extracts offer hepatoprotection against restraint stress-induced liver injury in mice by modulating mitochondrial dysfunction. Food Funct. 2016, 7, 508–515. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, R.; Liu, Y.; Xiao, J.; Liu, L.; Wei, Z.; Yi, Y.; Zhang, M.; Liu, D. Dietary litchi pulp polysaccharides could enhance immunomodulatory and antioxidant effects in mice. Int. J. Biol. Macromol. 2016, 92, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Zhang, R.; Hou, F.; Zhang, M.; Guo, J.; Huang, F.; Deng, Y.; Wei, Z. Comparison of the free and bound phenolic profiles and cellular antioxidant activities of litchi pulp extracts from different solvents. BMC Complement. Altern. Med. 2014, 14, 9. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Wang, K.; Wang, K.; Zhu, J.; Hu, Z. Nutrient components, health benefits, and safety of litchi (Litchi chinensis Sonn.): A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2139–2163. [Google Scholar] [CrossRef]
- Perry, J.R.; Ying, W. A Review of Physiological Effects of Soluble and Insoluble Dietary Fibers. A Review of Physiological Effects of Soluble and Insoluble Dietary Fibers. J. Nutr. Food Sci. 2016, 6, 476. [Google Scholar] [CrossRef]
- Fuller, S.; Beck, E.; Salman, H.; Tapsell, L. New Horizons for the Study of Dietary Fiber and Health: A Review. Plant Foods Hum. Nutr. 2016, 71, 1–12. [Google Scholar] [CrossRef]
- Ain, H.B.U.; Saeed, F.; Ahmed, A.; Khan, M.A.; Niaz, B.; Tufail, T. Improving the physicochemical properties of partially enhanced soluble dietary fiber through innovative techniques: A coherent review. J. Food Process. Preserv. 2019, 43, e13917. [Google Scholar] [CrossRef]
- Ozyurt, V.H.; Ötles, S. Effect of food processing on the physicochemical properties of dietary fibre. Acta Sci. Pol. Technol. Aliment. 2016, 15, 233–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, J.; Xie, L.; Peng, G.; Xie, J.; Chen, Y.; Yu, Q. Systematic review on modification methods of dietary fiber. Food Hydrocoll. 2021, 119, 106872. [Google Scholar] [CrossRef]
- Wu, W.; Hu, J.; Gao, H.; Chen, H.; Fang, X.; Mu, H.; Han, Y.; Liu, R. The potential cholesterol-lowering and prebiotic effects of bamboo shoot dietary fibers and their structural characteristics. Food Chem. 2020, 332, 127372. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z.; Wang, Z.; Hao, Y.; Wang, Y.; Yang, Z.; Li, W.; Wang, J. Physicochemical and functional properties of soluble dietary fiber from different colored quinoa varieties (Chenopodium quinoa Willd). J. Cereal Sci. 2020, 95, 103045. [Google Scholar] [CrossRef]
- Ma, R.; Chen, J.-N.; Zhou, X.-J.; Lin, H.; Gao, Q.; Peng, X.; Tanokura, M.; Xue, Y.-L. Effect of chemical and enzymatic modifications on the structural and physicochemical properties of dietary fiber from purple turnip (Brassica rapa L.). LWT 2021, 145, 111313. [Google Scholar] [CrossRef]
- Shen, M.; Weihao, W.; Cao, L. Soluble dietary fibers from black soybean hulls: Physical and enzymatic modification, structure, physical properties, and cholesterol binding capacity. J. Food Sci. 2020, 85, 1668–1674. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Content of Insoluble Bound Phenolics in Millets and Their Contribution to Antioxidant Capacity. J. Agric. Food Chem. 2010, 58, 6706–6714. [Google Scholar] [CrossRef]
- Ruiz-Torralba, A.; Guerra-Hernández, E.J.; García-Villanova, B. Antioxidant capacity, polyphenol content and contribution to dietary intake of 52 fruits sold in Spain. CyTA -J. Food 2018, 16, 1131–1138. [Google Scholar] [CrossRef] [Green Version]
- Okolie, C.L.; Mason, B.; Mohan, A.; Pitts, N.; Udenigwe, C.C. The comparative influence of novel extraction technologies on in vitro prebiotic-inducing chemical properties of fucoidan extracts from Ascophyllum nodosum. Food Hydrocoll. 2018, 90, 462–471. [Google Scholar] [CrossRef]
- Akbari-Alavijeh, S.; Soleimanian-Zad, S.; Sheikh-Zeinoddin, M.; Hashmi, S. Pistachio hull water-soluble polysaccharides as a novel prebiotic agent. Int. J. Biol. Macromol. 2018, 107, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, L.; Huang, X.; Jing, H.; Ye, X.; Gao, W.; Bai, X.; Wang, H. Effects of different extraction methods on structure and properties of soluble dietary fiber from defatted coconut flour. LWT 2021, 143, 111031. [Google Scholar] [CrossRef]
- Yan, L.; Li, T.; Liu, C.; Zheng, L. Effects of high hydrostatic pressure and superfine grinding treatment on physicochemical/ functional properties of pear pomace and chemical composition of its soluble dietary fibre. LWT 2019, 107, 171–177. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Yuan, F.; Pan, Q.; Fan, R.; Gao, Y. Physicochemical characterization of five types of citrus dietary fibers. Biocatal. Agric. Biotechnol. 2015, 4, 250–258. [Google Scholar] [CrossRef]
- Hao, L.; Lu, X.; Sun, M.; Li, K.; Shen, L.; Wu, T. Protective effects of L-arabinose in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Food Nutr. Res. 2015, 59, 28886. [Google Scholar] [CrossRef] [Green Version]
- Tamura, M.; Kurusu, Y.; Hori, S. Effect of Dietary L-arabinose on the Intestinal Microbiota and Metabolism of Dietary Daidzein in Adult Mice. Biosci. Microbiota Food Heal. 2012, 31, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wandee, Y.; Uttapap, D.; Mischnick, P. Yield and structural composition of pomelo peel pectins extracted under acidic and alkaline conditions. Food Hydrocoll. 2019, 87, 237–244. [Google Scholar] [CrossRef]
- Qi, J.; Yokoyama, W.; Masamba, K.G.; Majeed, H.; Zhong, F.; Li, Y. Structural and physico-chemical properties of insoluble rice bran fiber: Effect of acid–base induced modifications. RSC Adv. 2015, 5, 79915–79923. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Wang, Y.; Liu, Z.; Ni, Y. Effects of extraction methods on the structural characteristics and functional properties of dietary fiber extracted from kiwifruit (Actinidia deliciosa). Food Hydrocoll. 2020, 110, 106162. [Google Scholar] [CrossRef]
- Lyu, B.; Wang, H.; Swallah, M.S.; Fu, H.; Shen, Y.; Guo, Z.; Tong, X.; Li, Y.; Yu, H.; Jiang, L. Structure, Properties and Potential Bioactivities of High-purity Insoluble Fibre from Soybean Dregs (Okara). Food Chem. 2021, 364, 130402. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiong, M.; Bai, T.; Chen, D.; Zhang, Q.; Lin, D.; Liu, Y.; Liu, A.; Huang, Z.; Qin, W. Comparative study on the structure, physicochemical, and functional properties of dietary fiber extracts from quinoa and wheat. LWT 2021, 149, 111816. [Google Scholar] [CrossRef]
- Wen, Y.; Niu, M.; Zhang, B.; Zhao, S.; Xiong, S. Structural characteristics and functional properties of rice bran dietary fiber modified by enzymatic and enzyme-micronization treatments. LWT–Food Sci. Technol. 2017, 75, 344–351. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, R.; Yin, L.; Zhang, N. Novel blasting extrusion processing improved the physicochemical properties of soluble dietary fiber from soybean residue and in vivo evaluation. J. Food Eng. 2014, 120, 1–8. [Google Scholar] [CrossRef]
- Lettow, M.; Grabarics, M.; Mucha, E.; Thomas, D.A.; Polewski, L.; Freyse, J.; Rademann, J.; Meijer, G.; Von Helden, G.; Pagel, K. IR action spectroscopy of glycosaminoglycan oligosaccharides. Anal. Bioanal. Chem. 2020, 412, 533–537. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Chen, J.; Chen, F.; Wang, X.; Zhu, Q.; Ao, Q. Surface characterization of corn stalk superfine powder studied by FTIR and XRD. Colloids Surf. B Biointerfaces 2013, 104, 207–211. [Google Scholar] [CrossRef]
- Li, N.; Feng, Z.; Niu, Y.; Yu, L. Structural, rheological and functional properties of modified soluble dietary fiber from tomato peels. Food Hydrocoll. 2018, 77, 557–565. [Google Scholar] [CrossRef]
- Alfredo, V.-O.; Gabriel, R.-R.; Luis, C.-G.; David, B.-A. Physicochemical properties of a fibrous fraction from chia (Salvia hispanica L.). LWT-Food Sci. Technol. 2008, 42, 168–173. [Google Scholar] [CrossRef]
- Zhu, K.; Huang, S.; Peng, W.; Qian, H.; Zhou, H. Effect of ultrafine grinding on hydration and antioxidant properties of wheat bran dietary fiber. Food Res. Int. 2010, 43, 943–948. [Google Scholar] [CrossRef]
- Rosell, C.M.; Santos, E.; Collar, C. Physico-chemical properties of commercial fibres from different sources: A comparative approach. Food Res. Int. 2008, 42, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Twarogowska, A.; Van Poucke, C.; Van Droogenbroeck, B. Upcycling of Belgian endive (Cichorium intybus var. foliosum) by-products. Chemical composition and functional properties of dietary fibre root powders. Food Chem. 2020, 332, 127444. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Dou, W.; Alaxi, S.; Niu, Y.; Yu, L.L. Modified soluble dietary fiber from black bean coats with its rheological and bile acid binding properties. Food Hydrocoll. 2017, 62, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Zhang, X.; Hong, Y.; Li, Z.; Li, C.; Gu, Z. Characterisation of physicochemical and functional properties of soluble dietary fibre from potato pulp obtained by enzyme-assisted extraction. Int. J. Biol. Macromol. 2017, 101, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Dhital, S.; Dolan, G.; Stokes, J.R.; Gidley, M.J. Enzymatic hydrolysis of starch in the presence of cereal soluble fibre polysaccharides. Food Funct. 2014, 5, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pu, J.; Liu, D.; Yu, W.; Shao, Y.; Yang, G.; Xiang, Z.; He, N. Anti-Inflammatory and Antinociceptive Properties of Flavonoids from the Fruits of Black Mulberry (Morus nigra L.). PLoS ONE 2016, 11, e0153080. [Google Scholar] [CrossRef]
- Deng, J.-S.; Chi, C.-S.; Huang, S.-S.; Shie, P.-H.; Lin, T.-H.; Huang, G.-J. Antioxidant, analgesic, and anti-inflammatory activities of the ethanolic extracts of Taxillus liquidambaricola. J. Ethnopharmacol. 2011, 137, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zeng, Q.; Deng, Y.; Zhang, M.; Wei, Z.; Zhang, Y.; Tang, X. Phenolic profiles and antioxidant activity of litchi pulp of different cultivars cultivated in Southern China. Food Chem. 2013, 136, 1169–1176. [Google Scholar] [CrossRef]
- Guo, W.; Beta, T. Phenolic acid composition and antioxidant potential of insoluble and soluble dietary fibre extracts derived from select whole-grain cereals. Food Res. Int. 2013, 51, 518–525. [Google Scholar] [CrossRef]
- Hu, S.; Yin, J.; Nie, S.; Wang, J.; Phillips, G.O.; Xie, M.; Cui, S.W. In vitro evaluation of the antioxidant activities of carbohydrates. Bioact. Carbohydr. Diet. Fibre 2016, 7, 19–27. [Google Scholar] [CrossRef]
- Chen, G.; Chen, X.; Yang, B.; Yu, Q.; Wei, X.; Ding, Y.; Kan, J. New insight into bamboo shoot (Chimonobambusa quadrangularis) polysaccharides: Impact of extraction processes on its prebiotic activity. Food Hydrocoll. 2019, 95, 367–377. [Google Scholar] [CrossRef]
- Tadayoni, M.; Sheikh-Zeinoddin, M.; Soleimanian-Zad, S. Isolation of bioactive polysaccharide from acorn and evaluation of its functional properties. Int. J. Biol. Macromol. 2015, 72, 179–184. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, J.; Tao, S.; Zhou, X.; Pi, Y.; Gerrits, W.J.; Johnston, L.J.; Zhang, S.; Yang, H.; Liu, L.; et al. Effect of dietary fiber fermentation on short-chain fatty acid production and microbial composition in vitro. J. Sci. Food Agric. 2020, 100, 4282–4291. [Google Scholar] [CrossRef] [PubMed]
- Linares, D.M.; Ross, P.; Stanton, C. Beneficial Microbes: The pharmacy in the gut. Bioengineered 2016, 7, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| mg/g | SDF | M-SDF | IDF | M-IDF |
|---|---|---|---|---|
| Mannose | 37.49 ± 0.16 a | 27.22 ± 0.69 b | 26.63 ± 1.27 b | 25.44 ± 1.29 b |
| Ribose | 0.74 ± 0.19 a | 0.16 ± 0.01 b | 0.41 ± 0.04 b | 0.25 ± 0.02 b |
| Rhamnose | 17.81 ± 0.13 a | 18.63 ± 0.65 a | 8.43 ± 0.49 b | 5.86 ± 0.18 c |
| Galacturonic acid | 111.87 ± 4.54 b | 133.27 ± 6.46 a | 25.38 ± 1.27 c | 11.27 ± 0.48 d |
| Glucose | 44.40 ± 1.87 c | 79.17 ± 0.55 a | 50.14 ± 0.98 b | 49.09 ± 1.88 b |
| Galactose | 41.26 ± 0.14 a | 30.73 ± 1.09 b | 20.55 ± 0.27 c | 15.75 ± 0.15 d |
| Xylose | 3.21 ± 0.06 d | 7.03 ± 0.10 c | 21.90 ± 0.38 a | 12.32 ± 0.86 b |
| Arabinose | 187.02 ± 2.17 b | 233.11 ± 1.84 a | 102.00 ± 0.47 c | 83.14 ± 0.39 d |
| mg/g | SDF | M-SDF | IDF | M-IDF |
|---|---|---|---|---|
| WHC (g/g) | 6.75 ± 0.09 c | 6.9 ± 0.13 c | 9.69 ± 0.2 a | 9.07 ± 0.09 b |
| SC (mL/g) | 8.63 ± 0.18 a | 7.75 ± 0.35 b | 6.65 ± 0.28 c | 7.18 ± 0.25 b,c |
| WS (%) | 83.1 ± 1.76 a | 78.34 ± 0.2 b | 10.22 ± 0.17 c | 9.29 ± 0.32 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yu, Y.; Wu, J.; Xu, Y.; Xiao, G.; Li, L.; Liu, H. Comparison the Structural, Physicochemical, and Prebiotic Properties of Litchi Pomace Dietary Fibers before and after Modification. Foods 2022, 11, 248. https://doi.org/10.3390/foods11030248
Li Y, Yu Y, Wu J, Xu Y, Xiao G, Li L, Liu H. Comparison the Structural, Physicochemical, and Prebiotic Properties of Litchi Pomace Dietary Fibers before and after Modification. Foods. 2022; 11(3):248. https://doi.org/10.3390/foods11030248
Chicago/Turabian StyleLi, Yina, Yuanshan Yu, Jijun Wu, Yujuan Xu, Gengsheng Xiao, Lu Li, and Haoran Liu. 2022. "Comparison the Structural, Physicochemical, and Prebiotic Properties of Litchi Pomace Dietary Fibers before and after Modification" Foods 11, no. 3: 248. https://doi.org/10.3390/foods11030248