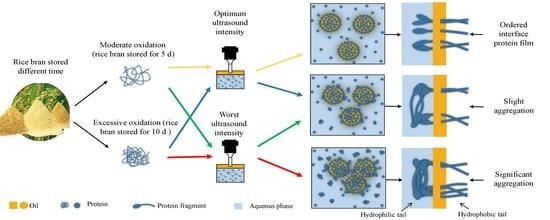
Strategy and Mechanism of Rice Bran Protein Emulsion Stability Based on Rancidity-Induced Protein Oxidation: An Ultrasonic Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. RBP Extraction
2.3. Measurement of RB Rancidity Extent and RBP Oxidation Extent
2.4. Preparation of RBPE
2.5. Determination of Emulsion Stability
2.5.1. Mean Droplet Diameter, Polydispersity Index, and Zeta Potential Determination of RBPE
2.5.2. Microstructure Measurement of RBPE
2.5.3. Creaming Index Measurement of RBPE
2.6. Extraction of Interface Protein
2.7. IAP Content Measurement
2.8. Methods for Determining the Structure of IAP and INP
2.8.1. Sulfhydryl and Disulfide Content Measurement
2.8.2. Fourier Transform Infrared Spectroscopy (FTIR) Measurement
2.8.3. Flexibility Measurement
2.8.4. Size Distribution and Zeta Potential Measurement
2.8.5. Surface Hydrophobicity Measurement
2.8.6. Intrinsic Fluorescence Measurement
2.8.7. Protein Electrophoresis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of RB Storage Time on the RB Rancidity Extent and RBP Oxidation Extent
3.2. Effect of Ultrasonic Treatment on the Stability of RBPE Prepared from RBP with Different Oxidation Extents
3.2.1. Analysis of MDD, Size Distribution, and Zeta Potential
3.2.2. Analysis of Macroscopic Stability and Microstructure
3.3. Effect of Ultrasonic Treatment on the Content and Structure of IAP and INP of RBPE Prepared from RBP with Different Oxidation Extents
3.3.1. Analysis of the IAP Content of RBPE Prepared from RBP with Different Oxidation Extents
3.3.2. Analysis of the Content of Free Sulfhydryl and Disulfide Bonds of IAP and INP of RBPE Prepared from RBP with Different Oxidation Extents
3.3.3. Analysis of FTIR of IAP and INP of RBPE Prepared from RBP with Different Extents of Oxidation
3.3.4. Analysis of Surface Hydrophobicity, Zeta Potential, and Protease Susceptibility of IAP and INP of RBPE Prepared from RBP with Different Oxidation Extents
3.3.5. Effect of Ultrasonic Treatment on the Spatial Structure of IAP and INP of RBPE Prepared from RBP with Different Oxidation Extents
Analysis of the Size Distribution and Intrinsic Fluorescence of IAP and INP of RBPE Prepared from RBP with Different Oxidation Extents
Analysis of the SDS-PAGE of IAP and INP of RBPE Prepared from RBP with Different Oxidation Extents
3.4. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, G.; Hu, M.; Lu, X.; Zhang, R. Soaking, heating and high hydrostatic pressure treatment degrade the flavonoids in rice bran. LWT–Food Sci. Technol. 2022, 154, 112732. [Google Scholar] [CrossRef]
- Bollinedi, H.; Singh, A.K.; Singh, N.; Gopala, K.S.; Bhowmick, P.K.; Vinod, K.K.; Nagarajan, M.; Ellur, R.K. Genetic and genomic approaches to address rapid rancidity of rice bran. CRC Crit. Rev. Food Technol. 2021, 61, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhao, Y.; Wang, X.; Tang, H.; Wu, N.; Wu, F.; Yu, D.; Elfalleh, W. Effects of (+)-catechin on a rice bran protein oil-in-water emulsion: Droplet size, zeta-potential, emulsifying properties, and rheological behavior. Food Hydrocoll. 2020, 98, 105306. [Google Scholar] [CrossRef]
- Najamuddin, U.; Gorji, S.G.; Fitzgerald, M. Genotypic variability in the composition of soluble protein from rice bran—Opportunities for nutrition. J. Food Compos. Anal. 2021, 103, 104077. [Google Scholar] [CrossRef]
- Li, H.; Li, F.; Wu, X.; Wu, W. Effect of rice bran rancidity on the emulsion stability of rice bran protein and structural characteristics of interface protein. Food Hydrocoll. 2021, 121, 107006. [Google Scholar] [CrossRef]
- Wu, W.; Li, F.; Wu, X. Effects of rice bran rancidity on oxidation, structural characteristics and interfacial properties of rice bran globulin. Food Hydrocoll. 2021, 110, 106123. [Google Scholar] [CrossRef]
- Zhao, Q.; Xie, T.; Hong, X.; Zhou, Y.; Fan, L.; Liu, Y.; Li, J. Modification of functional properties of perilla protein isolate by high-intensity ultrasonic treatment and the stability of o/w emulsion. Food Chem. 2022, 368, 130848. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, X.J.; Wu, W. Effects of malondialdehyde-induced protein oxidation on the structural characteristics of rice protein. Int. J. Food Sci. Technol. 2019, 55, 760–768. [Google Scholar] [CrossRef]
- Li, F.; Wu, X.J.; Wu, W. Effects of protein oxidation induced by rice bran rancidity on the structure and functionality of rice bran glutelin. LWT–Food Sci. Technol. 2021, 149, 111874. [Google Scholar] [CrossRef]
- Li, H.; Cai, Y.; Li, F.; Zhang, B.; Wu, X.; Wu, W. Rancidity-induced protein oxidation affects the interfacial dynamic properties and the emulsion rheological behavior of rice bran protein. Food Hydrocoll. 2022, 131, 107794. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Hernández-Ortega, C.; Welti-Chanes, J.; Putnik, P.; Barba, F.J.; Mallikarjunan, K.; Escobedo-Avellaneda, Z.; Roohinejad, S. High pressure processing of food-grade emulsion systems: Antimicrobial activity, and effect on the physicochemical properties. Food Hydrocoll. 2019, 87, 307–320. [Google Scholar] [CrossRef]
- Taha, A.; Ahmed, E.; Ismaiel, A.; Ashokkumar, M.; Xu, X.; Pan, S.; Hu, H. Ultrasonic emulsification: An overview on the preparation of different emulsifiers-stabilized emulsions. Trends Food Sci. Technol. 2020, 105, 363–377. [Google Scholar] [CrossRef]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Wang, J.Y.; Yang, Y.L.; Tang, X.Z.; Ni, W.X.; Zhou, L. Effects of pulsed ultrasound on rheological and structural properties of chicken myofibrillar protein. Ultrason. Sonochem. 2017, 38, 225–233. [Google Scholar] [CrossRef]
- Zhang, S.; Tian, L.; Yi, J.; Zhu, Z.; Dong, X.; Decker, E. Impact of high-intensity ultrasound on the chemical and physical stability of oil-in-water emulsions stabilized by almond protein isolate. LWT–Food Sci. Technol. 2021, 149, 111972. [Google Scholar] [CrossRef]
- Chen, W.; Ju, X.; Aluko, R.E.; Zou, Y.; Wang, Z.; Liu, M.; He, R. Rice bran protein-based nanoemulsion carrier for improving stability and bioavailability of quercetin. Food Hydrocoll. 2020, 108, 106042. [Google Scholar] [CrossRef]
- Yi, J.; Ning, J.; Zhu, Z.; Cui, L.; Decker, E.A.; McClements, D.J. Impact of interfacial composition on co-oxidation of lipids and proteins in oil-in-water emulsions: Competitive displacement of casein by surfactants. Food Hydrocoll. 2019, 87, 20–28. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, M.; Sun, W.; Ren, J.; Cui, C. Effect of oxidation on the emulsifying properties of soy protein isolate. Food Res. Int. 2013, 52, 26–32. [Google Scholar] [CrossRef]
- Liu, J.; Gao, T.; Li, F.; Xie, T. The addition of oxidized tea polyphenols enhances the physical and oxidative stability of rice bran protein hydrolysate-stabilized oil-in-water emulsions. Food Sci. Technol. Res. 2022, 28, 224–233. [Google Scholar] [CrossRef]
- Wang, W.; Wang, R.; Yao, J.; Luo, S.; Wang, X.; Zhang, N.; Wang, L.; Zhu, X. Effect of ultrasonic power on the emulsion stability of rice bran protein-chlorogenic acid emulsion. Ultrason. Sonochem. 2022, 84, 105959. [Google Scholar] [CrossRef] [PubMed]
- Nishad, J.; Dutta, A.; Saha, S.; Rudra, S.G.; Varghese, E.; Sharma, R.R.; Tomar, M.; Kumar, M.; Kaur, C. Ultrasound-assisted development of stable grapefruit peel polyphenolic nano-emulsion: Optimization and application in improving oxidative stability of mustard oil. Food Chem. 2021, 334, 127561. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Sun, R.; Shi, J.; Li, M.; Guan, X.; Liu, J.; Huang, K.; Zhang, Y. Effect of ultrasonic on the structure and quality characteristics of quinoa protein oxidation aggregates. Ultrason. Sonochem. 2021, 77, 105685. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.H.; Heo, Y.S.; Yim, D.G.; Lee, Y.E.; Kang, T.; Kim, H.-J.; Jo, C. Influence of atmospheric-pressure cold plasma-induced oxidation on the structure and functional properties of egg white protein. Innovative Food Sci. Emerging Technol. 2021, 74, 102869. [Google Scholar] [CrossRef]
- Wang, S.; Wang, T.; Li, X.; Cui, Y.; Sun, Y.; Yu, G.; Cheng, J. Fabrication of emulsions prepared by rice bran protein hydrolysate and ferulic acid covalent conjugate: Focus on ultrasonic emulsification. Ultrason. Sonochem. 2022, 88, 106064. [Google Scholar] [CrossRef]
- Wang, T.; Chen, K.; Zhang, X.; Yu, Y.; Yu, D.; Jiang, L.; Wang, L. Effect of ultrasound on the preparation of soy protein isolate-maltodextrin embedded hemp seed oil microcapsules and the establishment of oxidation kinetics models. Ultrason. Sonochem. 2021, 77, 105700. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Xue, S.; Xu, X. Effects of ultrasound frequency mode on myofibrillar protein structure and emulsifying properties. Int. J. Biol. Macromol. 2020, 163, 1768–1779. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Qin, W.; Gu, J.; Zhang, H.; Duan, Y.; Ma, H. Structure and functional properties of soy protein isolate-lentinan conjugates obtained in Maillard reaction by slit divergent ultrasonic assisted wet heating and the stability of oil-in-water emulsions. Food Chem. 2020, 331, 127374. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Bi, S.; Qi, B.; Wang, Z.; Zhang, M.; Li, Y.; Jiang, L. Impact of ultrasonic treatment on an emulsion system stabilized with soybean protein isolate and lecithin: Its emulsifying property and emulsion stability. Food Hydrocoll. 2017, 63, 727–734. [Google Scholar]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Wang, H.; Chen, Q.; Kong, B. High-intensity ultrasound improves the physical stability of myofibrillar protein emulsion at low ionic strength by destroying and suppressing myosin molecular assembly. Ultrason. Sonochem. 2021, 74, 105554. [Google Scholar] [CrossRef]
- Liu, C.; Pei, R.; Heinonen, M. Faba bean protein: A promising plant-based emulsifier for improving physical and oxidative stabilities of oil-in-water emulsions. Food Chem. 2022, 369, 130879. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Liang, M.; Yang, L. Glutelin and prolamin, different components of rice protein, exert differently in vitro antioxidant activities. J. Cereal Sci. 2016, 72, 108–116. [Google Scholar] [CrossRef]





| MDD(nm) | PDI | Zeta(mV) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 10 | 0 | 1 | 3 | 5 | 10 | 0 | 1 | 3 | 5 | 10 | |
| 300 W 20 min | 1954.33 ± 402.50 a | 1378.00 ± 112.79 b | 1831.00 ± 59.86 a | 505.80 ± 15.56 f | 1432.00 ± 17.83 a | 0.95 ± 0.06 a | 0.89 ± 0.08 ab | 0.96 ± 0.07 ab | 0.41 ± 0.06 c | 0.88 ± 0.03 a | −25.56 ± 1.20 a | −26.83 ± 0.67 a | −26.59 ± 1.49 ab | −29.37 ± 0.32 a | −26.66 ± 1.42 a |
| 300 W 30 min | 1594.60 ± 42.69 b | 1334.50 ± 55.54 bc | 1720.08 ± 122.17 ab | 727.53 ± 4.17 ef | 1404.00 ± 278.26 a | 0.89 ± 0.15 ab | 0.80 ± 0.17 bcd | 0.98 ± 0.09 a | 0.51 ± 0.06 c | 0.83 ± 0.11 ab | −26.44 ± 0.94 a | −27.80 ± 0.36 ab | −26.45 ± 1.45 ab | −28.87 ± 0.21 a | −27.17 ± 1.69 ab |
| 300 W 40 min | 1584.27 ± 426.38 b | 1304.87 ± 309.98 bcd | 1724.67 ± 48.21 ab | 1153.33 ± 16.04 bcd | 1391.85 ± 302.41 a | 0.88 ± 0.21 abc | 0.77 ± 0.13 bcde | 0.96 ± 0.06 ab | 0.70 ± 0.00 ab | 0.81 ± 0.08 ab | −28.28 ± 2.11 b | −27.70 ± 0.26 ab | −26.43 ± 1.52 ab | −28.47 ± 0.25 a | −27.39 ± 1.06 abc |
| 300 W 50 min | 1235.73 ± 126.87 c | 1298.00 ± 65.51 bcd | 1564.43 ± 195.86 bc | 1132.33 ± 20.01 bcd | 1350.67 ± 323.03 a | 0.85 ± 0.16 abc | 0.64 ± 0.19 ef | 1.00 ± 0.00 a | 0.78 ± 0.09 ab | 0.80 ± 0.18 ab | −28.37 ± 1.55 b | −27.82 ± 1.02 ab | −26.43 ± 1.59 ab | −28.30 ± 0.26 a | −27.96 ± 1.41 abcd |
| 400 W 20 min | 1187.33 ± 45.01 c | 821.73 ± 28.41 e | 1785.67 ± 19.55 a | 904.20 ± 31.89 de | 1336.00 ± 12.53 a | 0.86 ± 0.16 abc | 0.57 ± 0.08 fg | 1.00 ± 0.00 a | 0.66 ± 0.04 b | 0.80 ± 0.15 ab | −25.67 ± 0.79 a | −27.88 ± 0.96 ab | −25.49 ± 0.59 a | −28.48 ± 1.03 a | −28.22 ± 1.11 bcd |
| 400 W 30 min | 1502.60 ± 131.38 bc | 603.27 ± 23.03 f | 617.23 ± 28.30 e | 925.00 ± 48.75 de | 967.20 ± 37.70 b | 0.86 ± 0.12 abc | 0.44 ± 0.02 g | 0.59 ± 0.09 d | 0.71 ± 0.11 ab | 0.80 ± 0.10 ab | −26.48 ± 1.38 a | −28.60 ± 0.61 b | −28.13 ± 0.21 bc | −27.93 ± 0.35 a | −28.46 ± 1.54 bcd |
| 400 W 40 min | 1452.63 ± 283.42 bc | 739.54 ± 162.23 ef | 668.86 ± 102.19 e | 947.60 ± 28.28 cde | 952.40 ± 31.12 b | 0.78 ± 0.12 bc | 0.67 ± 0.11 def | 0.59 ± 0.08 d | 0.72 ± 0.09 ab | 0.79 ± 0.04 ab | −28.36 ± 0.87 b | −28.05 ± 1.49 ab | −29.10 ± 0.90 c | −27.73 ± 0.43 a | −28.59 ± 1.73 bcd |
| 400 W 50 min | 1217.50 ± 39.07 c | 1132.80 ± 77.62 d | 1425.78 ± 217.37 c | 1228.33 ± 21.03 bc | 832.35 ± 57.47 b | 0.83 ± 0.11 abc | 0.71 ± 0.17 def | 0.90 ± 0.10 b | 0.79 ± 0.16 ab | 0.78 ± 0.10 ab | −28.60 ± 1.42 b | −27.07 ± 0.67 ab | −25.67 ± 1.47 a | −27.72 ± 1.28 a | −28.74 ± 1.28 cd |
| 500 W 20 min | 1284.67 ± 59 bc | 1153.75 ± 48.49 cd | 1601.14 ± 245.24 bc | 1056.89 ± 163.13 bcd | 826.96 ± 25.50 b | 0.81 ± 0.08 abc | 0.73 ± 0.05 cde | 0.79 ± 0.07 c | 0.76 ± 0.15 ab | 0.71 ± 0.08 bc | −25.62 ± 1.12 a | −27.84 ± 0.87 ab | −25.56 ± 0.61 a | −27.86 ± 1.67 a | −28.74 ± 1.56 cd |
| 500 W 30 min | 1211.43 ± 411.29 c | 1408.33 ± 46.01 ab | 687.84 ± 52.45 e | 1123.29 ± 45.52 bcd | 752.90 ± 21.70 b | 0.74 ± 0.17 c | 0.78 ± 0.08 bcde | 0.62 ± 0.08 d | 0.80 ± 0.05 ab | 0.68 ± 0.08 bc | −26.53 ± 1.03 a | −27.70 ± 0.73 ab | −27.00 ± 1.15 ab | −27.56 ± 1.51 a | −28.84 ± 1.34 cd |
| 500 W 40 min | 853.88 ± 132.50 d | 1459.47 ± 102.79 ab | 641.66 ± 19.19 e | 1321.00 ± 82.40 b | 948.80 ± 30.25 b | 0.54 ± 0.09 d | 0.88 ± 0.1 abc | 0.57 ± 0.1 d | 0.83 ± 0.17 ab | 0.63 ± 0.04 cd | −29.22 ± 1.58 b | −27.51 ± 0.77 ab | −27.50 ± 0.20 bc | −27.59 ± 1.26 a | −29.11 ± 0.90 d |
| 500 W 50 min | 675.25 ± 26.07 d | 1578.43 ± 85.77 a | 943.17 ± 176.54 d | 1679.27 ± 343.73 a | 682.57 ± 16.55 b | 0.51 ± 0.03 d | 0.96 ± 0.01 a | 0.82 ± 0.09 c | 0.85 ± 0.20 a | 0.52 ± 0.02 d | −29.31 ± 1.00 b | −27.04 ± 1.64 ab | −27.14 ± 1.13 ab | −27.40 ± 0.80 a | −29.35 ± 0.33 d |
| Γ (mg/m2) | Free Sulfhydryl (nmol/mg) | Disulfide Bonds (nmol/mg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group A | Group B | IAP in Group A | INP in Group A | IAP in Group B | INP in Group B | IAP in Group A | INP in Group A | IAP in Group B | INP in Group B | |
| 0 | 11.95 ± 0.00 b | 24.45 ± 0.28 b | 39.91 ± 0.48 c | 34.70 ± 0.65 a | 24.14 ± 0.41 c | 20.59 ± 0.36 a | 32.38 ± 1.78 a | 20.95 ± 0.29 c | 19.22 ± 0.97 ab | 9.80 ± 0.36 e |
| 1 | 11.67 ± 0.01 c | 24.04 ± 0.68 b | 41.94 ± 0.69 b | 33.42 ± 0.50 a | 25.38 ± 0.54 b | 18.81 ± 0.67 b | 25.57 ± 5.04 b | 22.71 ± 0.11 b | 17.78 ± 0.48 c | 11.02 ± 0.63 d |
| 3 | 11.10 ± 0.02 c | 23.18 ± 0.12 c | 44.93 ± 1.07 a | 30.19 ± 0.91 b | 27.33 ± 0.46 a | 16.30 ± 0.62 c | 28.98 ± 1.17 ab | 23.30 ± 0.37 b | 16.54 ± 0.17 d | 13.25 ± 0.08 c |
| 5 | 10.88 ± 0.01 d | 22.49 ± 0.09 d | 41.56 ± 0.94 b | 29.34 ± 0.72 b | 25.77 ± 0.25 b | 13.90 ± 0.39 d | 30.30 ± 0.35 a | 24.59 ± 0.35 a | 18.53 ± 0.16 bc | 16.24 ± 0.47 b |
| 10 | 11.98 ± 0.01 a | 26.26 ± 0.15 a | 40.01 ± 0.87 c | 27.77 ± 0.71 c | 24.29 ± 0.49 c | 11.30 ± 0.34 e | 32.11 ± 0.25 a | 25.57 ± 1.07 a | 19.90 ± 0.43 a | 17.88 ± 0.11 a |
| MDD | PDI | RBPE Zeta-Potential | CI | ||
|---|---|---|---|---|---|
| IAP | α-helix/β-sheet | −0.87 ** | −0.85 ** | −0.78 ** | −0.86 ** |
| Zeta-potential | 0.87 ** | 0.84 ** | 0.77 ** | 0.79 ** | |
| Disulfide bonds | −0.69 * | −0.69 * | −0.83 ** | −0.68 ** | |
| Flexible | −0.94 ** | −0.95 ** | −0.89 ** | −0.87 ** | |
| Surface hydrophobicity | −0.82 ** | −0.77 ** | −0.70 ** | −0.79 * | |
| Particle size distribution | 0.83 ** | 0.83 ** | 0.84 ** | 0.78 ** | |
| λmax | −0.42 | −0.41 | −0.45 | −0.57 | |
| INP | α-helix/β-sheet | −0.93 ** | −0.94 ** | −0.92 ** | −0.89 ** |
| Zeta-potential | 0.93 ** | 0.92 ** | 0.86 * | 0.89 ** | |
| Disulfide bonds | −0.84 ** | −0.88 ** | −0.77 ** | −0.95 ** | |
| Flexible | −0.94 ** | −0.95 ** | −0.89 ** | −0.89 ** | |
| Surface hydrophobicity | −0.82 * | −0.76 * | −0.73* | −0.81 ** | |
| Particle size distribution | 0.65 * | 0.70 * | 0.77* | 0.54 | |
| λmax | −0.63 | −0.60 | −0.58 | −0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Li, H.; Li, F.; Zhang, B.; Wu, X.; Wu, W. Strategy and Mechanism of Rice Bran Protein Emulsion Stability Based on Rancidity-Induced Protein Oxidation: An Ultrasonic Case Study. Foods 2022, 11, 3896. https://doi.org/10.3390/foods11233896
Zhou Q, Li H, Li F, Zhang B, Wu X, Wu W. Strategy and Mechanism of Rice Bran Protein Emulsion Stability Based on Rancidity-Induced Protein Oxidation: An Ultrasonic Case Study. Foods. 2022; 11(23):3896. https://doi.org/10.3390/foods11233896
Chicago/Turabian StyleZhou, Qi, Helin Li, Fang Li, Benpeng Zhang, Xiaojuan Wu, and Wei Wu. 2022. "Strategy and Mechanism of Rice Bran Protein Emulsion Stability Based on Rancidity-Induced Protein Oxidation: An Ultrasonic Case Study" Foods 11, no. 23: 3896. https://doi.org/10.3390/foods11233896