Application of Lactose-Free Whey Protein to Greek Yogurts: Potential Health Benefits and Impact on Rheological Aspects and Sensory Attributes
Abstract
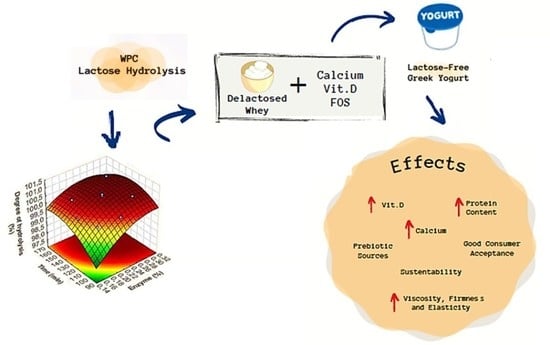
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physicochemical Characterization
2.3. Sugars and Lactic Acid Quantification
2.4. Experimental Planning
2.4.1. WPC Hydrolysis
2.4.2. Yogurt Formulation
2.5. Viscosity, Syneresis, Firmness, and Elasticity
2.6. Microbiological Analysis
2.7. Sensory Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. WPC Hydrolysis
3.2. Lactose-Free Yogurt Formulation
3.3. Microbiological Analysis
3.4. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Burgess, K. Milk and Dairy Products in Human Nutrition. Int. J. Dairy Technol. 2014, 67, 303–304. [Google Scholar] [CrossRef]
- Forsgård, R.A. Lactose Digestion in Humans: Intestinal Lactase Appears to Be Constitutive Whereas the Colonic Microbiome Is Adaptable. Am. J. Clin. Nutr. 2019, 110, 273–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enattah, N.S.; Sahi, T.; Savilahti, E.; Terwilliger, J.D.; Peltonen, L.; Järvelä, I. Identification of a Variant Associated with Adult-Type Hypolactasia. Nat. Genet. 2002, 30, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Bayless, T.M.; Brown, E.; Paige, D.M. Lactase Non-Persistence and Lactose Intolerance. Curr. Gastroenterol. Rep. 2017, 19, 23. [Google Scholar] [CrossRef]
- Dainese-Plichon, R.; Schneider, S.; Piche, T.; Hébuterne, X. Malabsorption et Intolérance Au Lactose Chez l’adulte. Nutr. Clin. métabolisme 2014, 28, 46–51. [Google Scholar] [CrossRef]
- Gulseven, O.; Wohlgenant, M. Demand for Functional and Nutritional Enhancements in Specialty Milk Products. Appetite 2014, 81, 284–294. [Google Scholar] [CrossRef]
- Almon, R.; Sjöström, M.; Nilsson, T.K. Lactase Non-Persistence as a Determinant of Milk Avoidance and Calcium Intake in Children and Adolescents. J. Nutr. Sci. 2013, 2, e26. [Google Scholar] [CrossRef] [Green Version]
- Szilagyi, A.; Galiatsatos, P.; Xue, X. Systematic Review and Meta-Analysis of Lactose Digestion, Its Impact on Intolerance and Nutritional Effects of Dairy Food Restriction in Inflammatory Bowel Diseases. Nutr. J. 2016, 15, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heine, R.G.; AlRefaee, F.; Bachina, P.; De Leon, J.C.; Geng, L.; Gong, S.; Madrazo, J.A.; Ngamphaiboon, J.; Ong, C.; Rogacion, J.M. Lactose Intolerance and Gastrointestinal Cow’s Milk Allergy in Infants and Children—Common Misconceptions Revisited. World Allergy Organ. J. 2017, 10, 41. [Google Scholar] [CrossRef] [Green Version]
- Ohlsson, J.A.; Johansson, M.; Hansson, H.; Abrahamson, A.; Byberg, L.; Smedman, A.; Lindmark-Månsson, H.; Lundh, Å. Lactose, Glucose and Galactose Content in Milk, Fermented Milk and Lactose-Free Milk Products. Int. Dairy J. 2017, 73, 151–154. [Google Scholar] [CrossRef]
- Moreira, T.C.; Transfeld da Silva, Á.; Fagundes, C.; Ferreira, S.M.R.; Cândido, L.M.B.; Passos, M.; Krüger, C.C.H. Elaboration of Yogurt with Reduced Level of Lactose Added of Carob (Ceratonia siliqua L.). LWT—Food Sci. Technol. 2017, 76, 326–329. [Google Scholar] [CrossRef]
- Jørgensen, C.E.; Abrahamsen, R.K.; Rukke, E.-O.; Hoffmann, T.K.; Johansen, A.-G.; Skeie, S.B. Processing of High-Protein Yoghurt—A Review. Int. Dairy J. 2019, 88, 42–59. [Google Scholar] [CrossRef]
- Neves, L.N.; de Oliveira, M.A.L. Assessment of Enzymatic Hydrolysis of Lactose in Lactose-Free Milk Production—A Comparative Study Using Capillary Zone Electrophoresis and Cryoscopy. LWT 2021, 138, 110585. [Google Scholar] [CrossRef]
- Skryplonek, K.; Henriques, M.; Gomes, D.; Viegas, J.; Fonseca, C.; Pereira, C.; Dmytrów, I.; Mituniewicz-Małek, A. Characteristics of Lactose-Free Frozen Yogurt with κ-Carrageenan and Corn Starch as Stabilizers. J. Dairy Sci. 2019, 102, 7838–7848. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ye, A.; Singh, H. Effects of Seasonal Variations on the Quality of Set Yogurt, Stirred Yogurt, and Greek-Style Yogurt. J. Dairy Sci. 2020, 104, 1424–1432. [Google Scholar] [CrossRef]
- Bong, D.D.; Moraru, C.I. Use of Micellar Casein Concentrate for Greek-Style Yogurt Manufacturing: Effects on Processing and Product Properties. J. Dairy Sci. 2014, 97, 1259–1269. [Google Scholar] [CrossRef]
- Candido, L.M.B.; Krüger, C.C.H. Proteínas Do Soro de Leite Bovino. Composição, Propriedades Nutritivas e Funcionais Tecnológicas, Aplicações. In Inovação nos Processos de Obtenção, Purificação e Aplicação dos Componentes do Leite Bovino; Farfan, J.A., Amaya, D.R., Sgarbieri, V., Eds.; Editora Atheneu: São Paulo, Brazil, 2012; pp. 121–156. [Google Scholar]
- Krzeminski, A.; Großhable, K.; Hinrichs, J. Structural Properties of Stirred Yoghurt as Influenced by Whey Proteins. Lebenson. Wiss. Technol. 2011, 44, 2134–2140. [Google Scholar] [CrossRef]
- Rico, C.; Muñoz, N.; Fernández, J.; Rico, J.L. High-Load Anaerobic Co-Digestion of Cheese Whey and Liquid Fraction of Dairy Manure in a One-Stage UASB Process: Limits in Co-Substrates Ratio and Organic Loading Rate. Chem. Eng. J. 2015, 262, 794–802. [Google Scholar] [CrossRef]
- Mansor, E.S.; Ali, E.A.; Shaban, A.M. Tight Ultrafiltration Polyethersulfone Membrane for Cheese Whey Wastewater Treatment. Chem. Eng. J. 2021, 407, 127175. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International; AOAC: Gaithersburg, MD, USA, 2008. [Google Scholar]
- Essig, A.M.; Kleyn, D.H. Determination of Lactose in Milk: Comparison of Methods. J. Assoc. Off. Anal. Chem. 1983, 66, 1514–1516. [Google Scholar] [CrossRef]
- Mirani, A.; Goli, M. Optimization of Cupcake Formulation by Replacement of Wheat Flour with Different Levels of Eggplant Fiber Using Response Surface Methodology. Food Sci. Technol. 2022, 42, e52120. [Google Scholar] [CrossRef]
- Riener, J.; Noci, F.; Cronin, D.A.; Morgan, D.J.; Lyng, J.G. A Comparison of Selected Quality Characteristics of Yoghurts Prepared from Thermosonicated and Conventionally Heated Milks. Food Chem. 2010, 119, 1108–1113. [Google Scholar] [CrossRef]
- Sandoval-Castilla, O.; Lobato-Calleros, C.; Aguirre-Mandujano, E.; Vernon-Carter, E.J. Microstructure and Texture of Yogurt as Influenced by Fat Replacers. Int. Dairy J. 2004, 14, 151–159. [Google Scholar] [CrossRef]
- Bourne, M. Appendix III—Guidelines and Conditions for Testing Foods. In Food Texture and Viscosity, 2nd ed.; Bourne, M.C., Ed.; Academic Press: London, UK, 2002; pp. 353–368. ISBN 978-0-12-119062-0. [Google Scholar]
- Wehr, H.M.; Frank, J.F. Standard Methods for the Examination of Dairy Products; American Public Health Association: Washington, DC, USA, 2004; ISBN 978-0-87553-002-4. [Google Scholar]
- Sarkar, P.; Ghosh, S.; Dutta, S.; Sen, D.; Bhattacharjee, C. Effect of Different Operating Parameters on the Recovery of Proteins from Casein Whey Using a Rotating Disc Membrane Ultrafiltration Cell. Desalination 2009, 249, 5–11. [Google Scholar] [CrossRef]
- Husain, Q. Beta Galactosidases and Their Potential Applications: A Review. Crit. Rev. Biotechnol. 2010, 30, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Vénica, C.I.; Perotti, M.C.; Bergamini, C.V. Organic Acids Profiles in Lactose-Hydrolyzed Yogurt with Different Matrix Composition. Dairy Sci. Technol. 2014, 94, 561–580. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Lactose Thresholds in Lactose Intolerance and Galactosaemia. EFSA J. 2010, 8, 1777. [Google Scholar] [CrossRef]
- Agencia Nacional de Vigilância Sanitária. Aprova o Regulamento Técnico Sobre Padrões Microbiológicos Para Alimentos; RDC 12/2001; ANVISA: Brasília, Brazil, 2001.
- Hossain, M.K.; Keidel, J.; Hensel, O.; Diakité, M. The Impact of Extruded Microparticulated Whey Proteins in Reduced-Fat, Plain-Type Stirred Yogurt: Characterization of Physicochemical and Sensory Properties. LWT 2020, 134, 109976. [Google Scholar] [CrossRef]
- Berber, M.; González-Quijano, G.K. Whey Protein Concentrate as a Substitute for Non-Fat Dry Milk in Yogurt. J. Food Process. Technol. 2015, 6, 1000530. [Google Scholar]
- Seckin, A.; Ozkilinc, A. Effect of Some Prebiotics Usage on Quality Properties of Concentrated Yogurt. J. Anim. Vet. Adv. 2011, 10, 1117–1123. [Google Scholar] [CrossRef]
- World Health Organization and Food and Agriculture Organizationof the United Nations. Codex Alimentarius. Milk and Milk Products, 2nd ed.; World Health Organization and Food and Agriculture Organizationof the United Nations: Rome, Italy, 2011.
- Bierzuńska, P.; Cais-Sokolińska, D.; Yiğit, A. Storage Stability of Texture and Sensory Properties of Yogurt with the Addition of Polymerized Whey Proteins. Foods 2019, 8, 548. [Google Scholar] [CrossRef] [PubMed]
- Hulmi, J.J.; Lockwood, C.M.; Stout, J.R. Effect of Protein/Essential Amino Acids and Resistance Training on Skeletal Muscle Hypertrophy: A Case for Whey Protein. Nutr. Metab. 2010, 7, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamime, A.Y.; Robinson, R.K. Tamime and Robinson’s Yoghurt; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Serafeimidou, A.; Zlatanos, S.; Laskaridis, K.; Sagredos, A. Chemical Characteristics, Fatty Acid Composition and Conjugated Linoleic Acid (CLA) Content of Traditional Greek Yogurts. Food Chem. 2012, 134, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Heaney, R.P. (Eds.) Calcium in Human Health; Nutrition and Health; Humana Press: Totowa, NJ, USA, 2006; ISBN 978-1-58829-452-4. [Google Scholar]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.P.; Bhardwaj, S.; Dhanjal, D.S.; Nepovimova, E.; Cruz-Martins, N.; Kuča, K.; Chopra, C.; Singh, R.; Kumar, H.; Șen, F.; et al. Plant Prebiotics and Their Role in the Amelioration of Diseases. Biomolecules 2021, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.T.; Vasconcelos, Q.D.J.S.; Abreu, G.C.; Albuquerque, A.O.; Vilar, J.L.; Aragão, G.F. Systematic Review of the Ingestion of Fructooligosaccharides on the Absorption of Minerals and Trace Elements versus Control Groups. Clin. Nutr. ESPEN 2021, 41, 68–76. [Google Scholar] [CrossRef]
- Ojwach, J.; Adetunji, A.I.; Mutanda, T.; Mukaratirwa, S. Oligosaccharides Production from Coprophilous Fungi: An Emerging Functional Food with Potential Health-Promoting Properties. Biotechnol. Rep. 2022, 33, e00702. [Google Scholar] [CrossRef]
- Ministério da Agricultura Pecuária e Abastecimento. Regulamento Técnico de Identidade e Qualidade de Leites Fermentados; IN 46/2007; MAPA: Brasilia, Brazil, 2007.
- Haase, G.; Jelen, P. Lactose: Crystallization, Hydrolysis and Value-Added Derivatives. Int. Dairy J. 2008, 18, 685–694. [Google Scholar] [CrossRef]

| Factors | Code | −1.41 | −1 | 0 | 1 | 1.41 |
|---|---|---|---|---|---|---|
| Lactose hydrolysis | ||||||
| Enzyme (%) | x1 | 0.159 | 0.18 | 0.23 | 0.28 | 0.30 |
| Time (min) | x2 | 101.7 | 110 | 130 | 150 | 158.28 |
| Factors | Code | −1.41 | −1 | 0 | 1 | 1.41 |
|---|---|---|---|---|---|---|
| Yogurt formulation | ||||||
| LFWPC (%) | x1 | 2.585 | 3 | 4 | 5 | 5.414 |
| LFPM (%) | x2 | 2.585 | 3 | 4 | 5 | 5.414 |
| Results | |
|---|---|
| Lactose (g 100 g−1) | 22.96 ± 0.07 |
| Glucose (g 100 g−1) | 0.62 ± 0.01 |
| Galactose (g 100 g−1) | 0.69 ± 0.02 |
| pH | 6.05 ± 0.01 |
| Acidity (g lactic acid 100 g−1) | 0.67 ± 0.00 |
| Fat (g 100 g−1) | 5.50 ± 0.00 |
| Protein (g 100 g−1) | 60.07 ± 0.08 |
| Moisture (%) | 5.64 ± 0.40 |
| Ashes (%) | 3.94 ± 0.03 |
| Formulas | Viscosity (mPa s) | Firmness (N) | Elasticity (mm) | Syneresis (%) |
|---|---|---|---|---|
| Control sample | 352.00 ± 2.64 i | 0.03 ± 0.00 g | 8.36 ± 0.22 ghij | 54.91 ± 1.69 a |
| 1 | 726.16 ± 2.46 e | 0.07 ± 0.00 f | 9.00 ± 0.03 efj | 1.75 ± 0.00 b |
| 2 | 739.00 ± 1.00 d | 0.07 ± 0.00 f | 9.27 ± 0.12 cdefh | 1.39 ± 0.6 b |
| 3 | 716.00 ± 1.73 f | 0.08 ± 0.00 de | 9.29 ± 0.37 cdefg | 1.16 ± 1.00 b |
| 4 | 886.00 ± 3.60 a | 0.1 ± 0.00 ab | 10.43 ± 0.07 b | 0.11 ± 0.19 b |
| 5 | 674.66 ± 4.16 g | 0.09 ± 0.00 ce | 9.24 ± 0.38 cdef | 2.07 ± 0.52 b |
| 6 | 854.66 ± 3.51 b | 0.1 ± 0.00 a | 12.12 ± 0.83 a | 0.11 ± 0.20 b |
| 7 | 666.00 ± 2.64 h | 0.07 ± 0.00 f | 9.57 ± 0.33 bf | 0.57 ± 0.99 b |
| 8 | 862.33 ± 2.30 b | 0.1 ± 0.00 a | 10.27 ± 0.49 bc | 0.00 ± 0.00 b |
| 9 | 776.00 ± 1.00 c | 0.09 ± 0.00 cd | 9.88 ± 0.16 be | 0.11 ± 0.20 b |
| 10 | 768.33 ± 3.51 c | 0.09 ± 0.00 bc | 10.17 ± 0.45 bd | 0.11 ± 0.20 b |
| Formula 4 | Formula 6 | Formula 8 | |
|---|---|---|---|
| Lactose (g 100 g−1) | 0.0 | 0.0 | 0.0 |
| Glucose (g 100 g−1) | 1.47 ± 0.08 a | 1.25 ± 0.18 a | 1.26 ± 0.05 a |
| Galactose (g 100 g−1) | 1.65 ± 0.31 a | 1.56 ± 0.28 a | 1.71 ± 0.07 a |
| Lactic Acid (g 100 g−1) | 1.30 ± 0.01 a | 1.49 ± 0.03 a | 1.46 ± 0.09 a |
| pH | 4.81 ± 0.01 a | 4.79 ± 0.00 a | 4.79 ± 0.00 a |
| Protein (g 100 g−1) | 8.04 ± 0.70 a | 7.94 ± 0.39 a | 7.41 ± 0.40 a |
| Fat (g 100 g−1) | 6.05 ± 1.01 a | 5.26 ± 0.83 a | 4.51 ± 0.87 a |
| Ashes (g 100 g−1) | 1.39 ± 0.06 a | 1.38 ± 0.06 a | 1.37 ± 0.16 a |
| Calcium (mg 100 g−1) | 215.16 ± 1.94 a | 220.05 ± 5.42 a | 155.68 ± 5.27 b |
| Total fibers (g 100 g−1) | 3.05 ± 0.05 a | 3.02 ± 0.04 a | 3.00 ± 0.03 a |
| Moisture (%) | 79.35 ± 0.37 a | 79.25 ± 0.28 a | 79.29 ± 0.29 a |
| Total solids (g 100 g−1) | 20.86 ± 0.37 a | 20.74 ± 0.28 a | 20.70 ± 0.29 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, A.T.; de Lima, J.J.; Reis, P.; Passos, M.; Baumgartner, C.G.; Sereno, A.B.; Krüger, C.C.H.; Cândido, L.M.B. Application of Lactose-Free Whey Protein to Greek Yogurts: Potential Health Benefits and Impact on Rheological Aspects and Sensory Attributes. Foods 2022, 11, 3861. https://doi.org/10.3390/foods11233861
da Silva AT, de Lima JJ, Reis P, Passos M, Baumgartner CG, Sereno AB, Krüger CCH, Cândido LMB. Application of Lactose-Free Whey Protein to Greek Yogurts: Potential Health Benefits and Impact on Rheological Aspects and Sensory Attributes. Foods. 2022; 11(23):3861. https://doi.org/10.3390/foods11233861
Chicago/Turabian Styleda Silva, Agatha Transfeld, Jair José de Lima, Priscila Reis, Maurício Passos, Catiucia Giraldi Baumgartner, Aiane Benevide Sereno, Cláudia Carneiro Hecke Krüger, and Lys Mary Bileski Cândido. 2022. "Application of Lactose-Free Whey Protein to Greek Yogurts: Potential Health Benefits and Impact on Rheological Aspects and Sensory Attributes" Foods 11, no. 23: 3861. https://doi.org/10.3390/foods11233861