1H NMR Metabolic Profiling and Meat Quality in Three Beef Cattle Breeds from Northeastern Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Meat Sample Preparation
2.2. Analysis of Meat Quality
2.2.1. pH Measurement
2.2.2. Instrumental Color Measurement
2.2.3. Water Holding Capacity (WHC) Measurement
2.2.4. Textural Properties of Beef
2.2.5. Macronutrients of Beef
2.2.6. Electronics Nose (E-Nose)
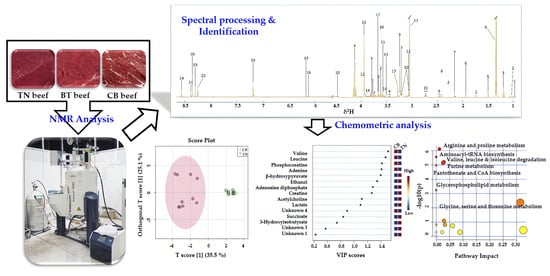
2.3. Metabolite Extraction, 1H NMR Analysis, Data Pre-Processing, and Metabolite Identification
2.4. Statistical Analysis
3. Results and Discussion
3.1. Beef Quality
3.2. H-NMR Metabolic Profiling of Beef
3.3. Multivariate Statistical Analysis and Pathways Analysis of Metabolites
3.4. Relationship of Metabolites and Meat Quality
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bertram, H.C.; Andersen, H.J. Proton NMR Relaxometry in Meat Science. In Modern Magnetic Resonance; Webb, G.A., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 1729–1733. ISBN 978-1-4020-3910-2. [Google Scholar]
- Khan, M.A.; Ahmad, B.; Kamboh, A.A.; Qadeer, Z.; Khan, M.A.; Ahmad, B.; Kamboh, A.A.; Qadeer, Z. Use of NMR relaxometry for determination of meat properties: A brief review. Food Mater. Res. 2022, 2, 8. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, M. Recent developments in the food quality detected by non-invasive nuclear magnetic resonance technology. Crit. Rev. Food Sci. Nutr. 2018, 59, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.B.; Kemp, R.; Samuelsson, L.M. Effects of dry-aging on meat quality attributes and metabolite profiles of beef loins. Meat Sci. 2016, 111, 168–176. [Google Scholar] [CrossRef]
- Kodani, Y.; Miyakawa, T.; Komatsu, T.; Tanokura, M. NMR-based metabolomics for simultaneously evaluating multiple determinants of primary beef quality in Japanese Black cattle. Sci. Rep. 2017, 7, 1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, S.F.; Farrell, D.; Kennedy, T.; Gordon, A.; Farmer, L.; Elliott, C.; Moss, B. Comparing GC–MS, HPLC and 1H NMR analysis of beef longissimus dorsi tissue extracts to determine the effect of suspension technique and ageing. Food Chem. 2012, 134, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Castejón, D.; García-Segura, J.M.; Escudero, R.; Herrera, A.; Cambero, M.I. Metabolomics of meat exudate: Its potential to evaluate beef meat conservation and aging. Anal. Chim. Acta 2015, 901, 1–11. [Google Scholar] [CrossRef]
- Ma, D.; Kim, Y.H.B.; Cooper, B.; Oh, J.-H.; Chun, H.; Choe, J.-H.; Schoonmaker, J.P.; Ajuwon, K.; Min, B. Metabolomics Profiling to Determine the Effect of Postmortem Aging on Color and Lipid Oxidative Stabilities of Different Bovine Muscles. J. Agric. Food Chem. 2017, 65, 6708–6716. [Google Scholar] [CrossRef]
- Muroya, S.; Oe, M.; Ojima, K.; Watanabe, A. Metabolomic approach to key metabolites characterizing postmortem aged loin muscle of Japanese Black (Wagyu) cattle. Asian-Australas. J. Anim. Sci. 2019, 32, 1172–1185. [Google Scholar] [CrossRef] [Green Version]
- Antonelo, D.; Gómez, J.F.M.; Cônsolo, N.R.B.; Beline, M.; Colnago, L.A.; Schilling, W.; Zhang, X.; Suman, S.P.; Gerrard, D.E.; Balieiro, J.C.C.; et al. Metabolites and Metabolic Pathways Correlated With Beef Tenderness. Meat Muscle Biol. 2020, 4, 19–20. [Google Scholar] [CrossRef]
- Antonelo, D.S.; dos Santos-Donado, P.R.; Ferreira, C.R.; Colnago, L.A.; Ocampos, F.M.M.; Ribeiro, G.H.; Ventura, R.V.; Gerrard, D.E.; Delgado, E.F.; Contreras-Castillo, C.J.; et al. Exploratory lipidome and metabolome profiling contributes to understanding differences in high and normal ultimate pH beef. Meat Sci. 2022, 194, 108978. [Google Scholar] [CrossRef]
- Zanardi, E.; Caligiani, A.; Palla, L.; Mariani, M.; Ghidini, S.; Di Ciccio, P.A.; Palla, G.; Ianieri, A. Metabolic profiling by 1H NMR of ground beef irradiated at different irradiation doses. Meat Sci. 2015, 103, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Lee, J.; Kwon, J.; Lee, K.-S.; Ryu, D.H.; Hwang, G.-S. Discrimination of the Geographical Origin of Beef by 1 H NMR-Based Metabolomics. J. Agric. Food Chem. 2010, 58, 10458–10466. [Google Scholar] [CrossRef]
- Trivedi, D.K.; Hollywood, K.A.; Rattray, N.J.W.; Ward, H.; Trivedi, D.K.; Greenwood, J.; Ellis, D.I.; Goodacre, R. Meat, the metabolites: An integrated metabolite profiling and lipidomics approach for the detection of the adulteration of beef with pork. Analyst 2016, 141, 2155–2164. [Google Scholar] [CrossRef] [Green Version]
- Ueda, S.; Iwamoto, E.; Kato, Y.; Shinohara, M.; Shirai, Y.; Yamanoue, M. Comparative metabolomics of Japanese Black cattle beef and other meats using gas chromatography–mass spectrometry. Biosci. Biotechnol. Biochem. 2019, 83, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Jakes, W.; Gerdova, A.; Defernez, M.; Watson, A.D.; McCallum, C.; Limer, E.; Colquhoun, I.J.; Williamson, D.C.; Kemsley, E.K. Authentication of beef versus horse meat using 60 MHz 1H NMR spectroscopy. Food Chem. 2015, 175, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Department of Livestock Development Information Regarding Animal Husbandry and Beef Cattle Farmers by Province for the 2021 Fiscal Year. Available online: http://ict.dld.go.th/webnew/images/stories/stat_web/yearly/2564/country/2-cattle.pdf (accessed on 30 July 2022).
- Wangkumhang, P.; Wilantho, A.; Shaw, P.J.; Flori, L.; Moazami-Goudarzi, K.; Gautier, M.; Duangjinda, M.; Assawamakin, A.; Tongsima, S. Genetic analysis of Thai cattle reveals a Southeast Asian indicine ancestry. PeerJ 2015, 3, e1318. [Google Scholar] [CrossRef] [Green Version]
- Sethakul, J.; Opatpatanakit, Y.; Sivapirunthep, P.; Intrapornudom, P. Beef Quality under Production Systems in Thailand: Preliminary Remarks. In Proceedings of the 13th AAAP Animal Science Congress, Hanoi, Vietnam, 22–26 September 2008. [Google Scholar]
- Bunmee, T.; Chaiwang, N.; Kaewkot, C.; Jaturasitha, S. Current situation and future prospects for beef production in Thailand—A review. Asian-Australas. J. Anim. Sci. 2018, 31, 968–975. [Google Scholar] [CrossRef] [PubMed]
- AMSA Meat Color Measurement Guidelines; American Meat Science Association: Champaign, IL, USA, 2012.
- AMSA. Research Guidelines for Cookery, Sensory Evaluation, and Instrumental Tenderness Measurements of Meat, 2nd ed.; American Meat Science Association: Champaign, IL, USA, 2016. [Google Scholar]
- Phoemchalard, C.; Tathong, T.; Pornanek, P.; Uriyapongson, S.; Cherdthong, A. Quality attributes of buffalo meat using precision sous vide cooking device. ARPN J. Eng. Appl. Sci. 2021, 16, 2117–2125. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Method of Analysis, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Cloarec, O.; Dumas, M.E.; Craig, A.; Barton, R.H.; Trygg, J.; Hudson, J.; Blancher, C.; Gauguier, D.; Lindon, J.C.; Holmes, E.; et al. Statistical Total Correlation Spectroscopy: An Exploratory Approach for Latent Biomarker Identification from Metabolic 1H NMR Data Sets. Anal. Chem. 2005, 77, 1282–1289. [Google Scholar] [CrossRef]
- SAS. SAS/STAT® 14.1 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2015. [Google Scholar]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- van den Berg, R.A.; Hoefsloot, H.C.J.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, L.; Jaworska, J.; Worth, A.P.; Cronin, M.T.D.; McDowell, R.M.; Gramatica, P. Methods for reliability and uncertainty assessment and for applicability evaluations of classification- and regression-based QSARs. Environ. Health Perspect. 2003, 111, 1361–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, R.K. 3—Factors affecting the quality of raw meat. In Meat Processing; Kerry, J., Kerry, J., Ledward, D., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2002; pp. 27–63. ISBN 978-1-85573-583-5. [Google Scholar]
- Page, J.K.; Wulf, D.M.; Schwotzer, T.R. A survey of beef muscle color and pH. J. Anim. Sci. 2001, 79, 678–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Phoemchalard, C.; Uriyapongson, S. Effect of cassava bioethanol by-product and crude palm oil in Brahman x Thai native yearling heifer cattle diets: II. Carcass characteristics and meat quality. Trop. Anim. Health Prod. 2015, 47, 1629–1631. [Google Scholar] [CrossRef]
- Chaosap, C.; Sivapirunthep, P.; Sitthigripong, R.; Tavitchasri, P.; Maduae, S.; Kusee, T.; Setakul, J.; Adeyemi, K. Meat quality, post-mortem proteolytic enzymes, and myosin heavy chain isoforms of different Thai native cattle muscles. Anim. Biosci. 2021, 34, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, S.F.; Tatum, J.D.; Wulf, D.M.; Green, R.D.; Smith, G.C. Genetic effects on beef tenderness in Bos indicus composite and Bos taurus cattle. J. Anim. Sci. 1997, 75, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Belew, J.B.; Brooks, J.C.; McKenna, D.R.; Savell, J.W. Warner–Bratzler shear evaluations of 40 bovine muscles. Meat Sci. 2003, 64, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Chaosap, C.; Lukkananukool, A.; Polyorach, S.; Sommart, K.; Sivapirunthep, P.; Limsupavanich, R. Effects of Dietary Energy Density in a Fermented Total Mixed Ration Formulated with Different Ratios of Rice Straw and Cassava Pulp on 2- or 14-Day-Aged Meat Quality, Collagen, Fatty Acids, and Ribonucleotides of Native Thai Cattle Longissimus Muscle. Foods 2022, 11, 2046. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.L.; Koohmaraie, M.; Cundiff, L.V.; Dikeman, M.E. Effects of cooking and shearing methodology on variation in Warner-Bratzler shear force values in beef. J. Anim. Sci. 1994, 72, 2325–2330. [Google Scholar] [CrossRef] [PubMed]
- Maltin, C.; Balcerzak, D.; Tilley, R.; Delday, M. Determinants of meat quality: Tenderness. Proc. Nutr. Soc. 2003, 62, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Warner, R.D.; Wheeler, T.L.; Ha, M.; Li, X.; Bekhit, A.E.-D.; Morton, J.; Vaskoska, R.; Dunshea, F.R.; Liu, R.; Purslow, P.; et al. Meat tenderness: Advances in biology, biochemistry, molecular mechanisms and new technologies. Meat Sci. 2022, 185, 108657. [Google Scholar] [PubMed]
- Malheiros, J.M.; Enríquez-Valencia, C.E.; Braga, C.P.; Vieira, J.C.S.; Vieira, D.S.; Pereira, G.L.; Curi, R.A.; Neto, O.R.M.; Oliveira, H.N.; Padilha, P.M.; et al. Application of proteomic to investigate the different degrees of meat tenderness in Nellore breed. J. Proteom. 2021, 248, 104331. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, A.; Panpipat, W.; Kim, S.R.; Chaijan, M. Characteristics of Thai Native Beef Slaughtered by Traditional Halal Method. Walailak J. Sci. Technol. 2018, 16, 443–453. [Google Scholar] [CrossRef]
- Waritthitham, A.; Lambertz, C.; Langholz, H.J.; Wicke, M.; Gauly, M. Assessment of beef production from Brahman × Thai native and Charolais × Thai native crossbred bulls slaughtered at different weights. II: Meat quality. Meat Sci. 2010, 85, 196–200. [Google Scholar] [CrossRef]
- Sarno, R.; Triyana, K.; Sabilla, S.I.; Wijaya, D.R.; Sunaryono, D.; Fatichah, C. Detecting Pork Adulteration in Beef for Halal Authentication using an Optimized Electronic Nose System. IEEE Access 2020, 8, 221700–221711. [Google Scholar] [CrossRef]
- Rahman Wijaya, D. Mobile electronic nose architecture for beef quality detection based on internet of things technology. In Proceedings of the 2nd International Conference on Global Trends in Academic Research, Bali, Indonesia, 2–3 June 2014; Global Illuminators Publishing: Bandung, Indonesia, 2015. [Google Scholar]
- Wijaya, D.R.; Sarno, R.; Zulaika, E. DWTLSTM for electronic nose signal processing in beef quality monitoring. Sens. Actuators B Chem. 2021, 326, 128931. [Google Scholar] [CrossRef]
- Wijaya, D.R.; Sarno, R.; Daiva, A.F. Electronic nose for classifying beef and pork using Naïve Bayes. In Proceedings of the International Seminar on Sensor, Instrumentation, Measurement and Metrology (ISSIMM): Innovation for the Advancement and Competitiveness of the Nation 2017, Surabaya, Indonesia, 25–26 August 2017; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2017; pp. 104–108. [Google Scholar]
- Sarno, R.; Wijaya, D.R. Recent development in electronic nose data processing for beef quality assessment. Telkomnika (Telecommun. Comput. Electron. Control) 2019, 17, 337–348. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.L.; Gao, T.; Lin, M.; Wang, X.F.; Zhu, X.D.; Gao, F.; Zhou, G.H. Effects of dietary supplementation with creatine monohydrate during the finishing period on growth performance, carcass traits, meat quality and muscle glycolytic potential of broilers subjected to transport stress. Animal 2014, 8, 1955–1962. [Google Scholar] [CrossRef] [Green Version]
- Mora, L.; Sentandreu, M.Á.; Toldrá, F. Contents of creatine, creatinine and carnosine in porcine muscles of different metabolic types. Meat Sci. 2008, 79, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Nissen, P.M.; Young, J.F. Creatine monohydrate and glucose supplementation to slow- and fast-growing chickens changes the postmortem pH in pectoralis major. Poult. Sci. 2006, 85, 1038–1044. [Google Scholar] [CrossRef]
- Choe, J.H.; Choi, Y.M.; Lee, S.H.; Shin, H.G.; Ryu, Y.C.; Hong, K.C.; Kim, B.C. The relation between glycogen, lactate content and muscle fiber type composition, and their influence on postmortem glycolytic rate and pork quality. Meat Sci. 2008, 80, 355–362. [Google Scholar] [CrossRef]
- Jolliffe, I.T. Principal Component Analysis for Special Types of Data; Springer: New York, NY, USA, 2002. [Google Scholar]
- Kang, J.; Choi, M.-Y.; Kang, S.; Kwon, H.N.; Wen, H.; Lee, C.H.; Park, M.; Wiklund, S.; Kim, H.J.; Kwon, S.W.; et al. Application of a 1H nuclear magnetic resonance (NMR) metabolomics approach combined with orthogonal projections to latent structure-discriminant analysis as an efficient tool for discriminating between Korean and Chinese herbal medicines. J. Agric. Food Chem. 2008, 56, 11589–11595. [Google Scholar] [CrossRef]
- Jia, W.; Dong, X.; Shi, L.; Chu, X.; Chu, X. Discrimination of Milk from Different Animal Species by a Foodomics Approach Based on High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2020, 68, 6638–6645. [Google Scholar] [CrossRef] [PubMed]
- Segato, S.; Caligiani, A.; Contiero, B.; Galaverna, G.; Bisutti, V.; Cozzi, G. 1H NMR Metabolic Profile to Discriminate Pasture Based Alpine Asiago PDO Cheeses. Animals 2019, 9, 722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellassi, P.; Rocchetti, G.; Nocetti, M.; Lucini, L.; Masoero, F.; Morelli, L. A Combined Metabolomic and Metagenomic Approach to Discriminate Raw Milk for the Production of Hard Cheese. Foods 2021, 10, 109. [Google Scholar] [CrossRef]
- Scano, P.; Carta, P.; Ibba, I.; Manis, C.; Caboni, P. An untargeted metabolomic comparison of milk composition from sheep kept under different grazing systems. Dairy 2020, 1, 30–41. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Fang, C.; He, J.; Dai, Q.; Fang, R. Comparison of the meat metabolite composition of Linwu and Pekin ducks using 600 MHz 1H nuclear magnetic resonance spectroscopy. Poult. Sci. 2017, 96, 192–199. [Google Scholar] [CrossRef]
- Jankowiak, H.; Cebulska, A.; Bocian, M. The relationship between acidification (pH) and meat quality traits of polish white breed pigs. Eur. Food Res. Technol. 2021, 247, 2813–2820. [Google Scholar] [CrossRef]
- Hoffman, L.C.; Fisher, P. Comparison of the effects of different transport conditions and lairage times in a Mediterranean climate in South Africa on the meat quality of commercially crossbred Large white x Landrace pigs. J. S. Afr. Vet. Assoc. 2010, 81, 224–227. [Google Scholar] [CrossRef]
- Shen, L.; Lei, H.; Zhang, S.; Li, X.; Li, M.; Jiang, X.; Zhu, K.; Zhu, L. The comparison of energy metabolism and meat quality among three pig breeds. Anim. Sci. J. 2014, 85, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Liu, Y.; Yu, Q. Metabolomic approach to measuring quality of chilled chicken meat during storage. Poult. Sci. 2020, 99, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Kim, M.; Ji, S.Y.; Baek, Y.C.; Lee, S.; Oh, Y.K.; Reddy, K.E.; Seo, H.W.; Cho, S.; Lee, H.J. Metabolomics Analysis of the Beef Samples with Different Meat Qualities and Tastes. Food Sci. Anim. Resour. 2020, 40, 924. [Google Scholar] [CrossRef] [PubMed]



| Items | TN | BT | CB | p-Value |
|---|---|---|---|---|
| pH | 5.61 ± 0.09 | 5.56 ± 0.16 | 5.52 ± 0.05 | 0.253 |
| Meat color | ||||
| L* | 39.25 ± 3.27 a | 34.63 ± 3.36 b | 34.19 ± 3.13 b | 0.003 |
| a* | 15.16 ± 3.13 | 15.08 ± 1.89 | 14.87 ± 1.88 | 0.961 |
| b* | 8.27 ± 1.62 | 8.38 ± 0.60 | 8.78 ± 0.73 | 0.550 |
| C* | 17.33 ± 3.11 | 17.29 ± 1.63 | 17.27 ± 1.91 | 0.998 |
| h* | 28.98 ± 5.83 | 29.33 ± 3.82 | 30.70 ± 2.13 | 0.632 |
| Water holding capacity | ||||
| Drip loss (%) | 11.15 ± 2.12 | 9.89 ± 2.45 | 9.77 ± 1.31 | 0.256 |
| Cooking loss (%) | 26.78 ± 2.85 | 23.40 ± 4.77 | 23.05 ± 3.59 | 0.072 |
| Shear values | ||||
| Shear force (kg/cm2) | 4.33 ± 1.01 a | 3.97 ± 1.30 a | 2.30 ± 0.65 b | <0.001 |
| Work of shear (kg·s) | 10.85 ± 1.87 a | 9.64 ± 3.49 a | 5.19 ± 1.28 b | <0.001 |
| Texture profile analysis | ||||
| Hardness (g) | 1298.03 ± 611.26 a | 774.07 ± 260.01 b | 710.85 ± 203.36 b | 0.005 |
| Adhesiveness (g·s) | −4.43 ± 5.70 | −4.50 ± 4.02 | −10.35 ± 13.60 | 0.245 |
| Springiness | 0.13 ± 0.07 | 0.09 ± 0.01 | 0.12 ± 0.04 | 0.156 |
| Cohesiveness | 0.76 ± 0.27 | 0.70 ± 0.06 | 0.75 ± 0.08 | 0.644 |
| Gumminess | 995.86 ± 606.16 a | 536.02 ± 176.79 b | 550.60 ± 154.66 b | 0.016 |
| Chewiness | 149.71 ± 162.91 a | 48.31 ± 21.93 b | 66.33 ± 31.09 a,b | 0.060 |
| Resilience | 0.29 ± 0.05 b | 0.30 ± 0.04 a | 0.36 ± 0.06 a | 0.005 |
| Chemical composition | ||||
| Moisture (%) | 74.46 ± 0.32 a | 73.58 ± 0.39 b | 64.87 ± 1.37 c | <0.001 |
| Protein (%) | 21.99 ± 0.30 b | 23.17 ± 0.16 a | 20.57 ± 0.13 c | <0.001 |
| Fat (%) | 2.12 ± 0.13 b | 2.43 ± 0.30 b | 13.40 ± 1.73 a | <0.001 |
| Ash (%) | 1.43 ± 0.50 a | 0.82 ± 0.19 b | 1.17 ± 0.52 a,b | 0.014 |
| Items | TN | BT | CB | p-Value |
|---|---|---|---|---|
| MOS-1 (TGS 816) | 8.28 ± 1.47 a,b | 7.55 ± 1.61 b | 9.34 ± 2.15 a | 0.028 |
| MOS-2 (TGS 2600) | 8.97 ± 4.16 c | 17.78 ± 3.16 a | 12.68 ± 3.93 b | <0.001 |
| MOS-3 (TGS 823) | 24.35 ± 12.28 b | 41.68 ± 7.00 a | 30.71 ± 8.85 b | <0.001 |
| MOS-4 (TGS 2603) | 27.79 ± 13.01 | 32.85 ± 5.21 | 31.07 ± 5.66 | 0.399 |
| MOS-5 (TGS 826) | 17.88 ± 5.43 c | 31.83 ± 4.77 a | 23.28 ± 6.51 b | <0.001 |
| MOS-6 (TGS 2610) | 7.06 ± 2.22 b | 9.70 ± 1.47 a | 8.63 ± 1.53 a | 0.004 |
| MOS-7 (TGS 2620) | 15.31 ± 5.66 c | 24.34 ± 3.65 a | 19.61 ± 4.05 b | <0.001 |
| MOS-8 (TGS 2444) | 8.03 ± 1.25 | 9.54 ± 1.47 | 11.43 ± 1.29 | 0.131 |
| No. | Metabolite | Chemical Shift (Multiplicity) | p-Value | ||
|---|---|---|---|---|---|
| TN/BT | TN/CB | BT/CB | |||
| 1 | Leucine | 0.9516 (t); 1.725 (m); 3.727 (t) | 0.999 | <0.001 | <0.001 |
| 2 | Valine | 0.9917 (dd); 1.037 (d); 2.28 (m); 3.632 (d) | 0.558 | <0.001 | <0.001 |
| 3 | Ethanol | 1.1707 (t); 3.65 (q) | 0.835 | <0.001 | <0.001 |
| 4 | Lactate | 1.3281 (d); 4.103 (q) | 0.456 | 0.012 | <0.001 |
| 5 | Alanine | 1.485 (d); 3.772 (q) | 0.734 | 0.986 | 0.637 |
| 6 | Acetate | 1.931 (s) | 0.197 | 0.734 | 0.570 |
| 7 | Acetylcholine | 2.156 (s); 3.205 (s); 3.742 (t) | 0.187 | 0.005 | 0.252 |
| 8 | Succinate | 2.4064 (s) | 0.938 | 0.107 | 0.199 |
| 9 | Carnitine | 2.44 (dd); 3.239 (s); 3.424 (m) | 0.054 | 0.738 | 0.225 |
| 10 | Unknown 1 | 2.7038 (dt); 3.055 (dd); 3.195 (t); 4.454 (t); 7.188 (s); 8.35 (d) | 0.881 | 0.627 | 0.349 |
| 11 | Creatine | 3.0432 (s); 3.941 (s) | 0.763 | <0.001 | <0.001 |
| 12 | Phosphocreatine | 3.0638 (s); 3.974 (s) | 1.000 | <0.001 | <0.001 |
| 13 | Trimethylamine-N-oxide | 3.2764 (s) | 0.111 | 0.776 | 0.026 |
| 14 | Unknown 2 | 3.503 (s); 3.527 (m); 3.727 (m); 3.846 (m); 3.974 (m) | 0.106 | 0.364 | 0.005 |
| 15 | 3-Hydroxyisobutyrate | 1.055 (d); 3.5597 (dd); 3.643 (dd) | 0.746 | 0.104 | 0.361 |
| 16 | Glycine | 3.5782 (s) | 0.930 | 0.960 | 0.801 |
| 17 | β-hydroxypyruvate | 3.668 (s) | 0.998 | <0.001 | <0.001 |
| 18 | Unknown 3 | 6.1105 (d) | 0.080 | 0.335 | 0.003 |
| 19 | Adenosine diphosphate | 4.19 (m); 6.1549 (d); 8.30 (s); 8.567 (s) | 0.802 | <0.001 | <0.001 |
| 20 | Adenine | 8.2509 (s) | 0.954 | <0.001 | <0.001 |
| 21 | Unknown 4 | 8.3845 (s) | 0.008 | 0.049 | 0.713 |
| Metabolite | TN | BT | CB | p-Value |
|---|---|---|---|---|
| Leucine | 1.76 ± 0.87 b | 1.75 ± 0.45 b | 3.58 ± 0.43 a | <0.001 |
| Valine | 1.78 ± 0.84 b | 2.13 ± 0.50 b | 4.71 ± 0.84 a | <0.001 |
| Ethanol | 21.18 ± 2.79 a | 21.77 ± 2.63 a | 16.29 ± 1.22 b | <0.001 |
| Lactate | 521.18 ± 103.58 a | 575.09 ± 116.52 a | 384.22 ± 72.17 b | 0.001 |
| Alanine | 21.21 ± 3.65 | 19.94 ± 5.03 | 21.48 ± 2.00 | 0.627 |
| Acetate | 13.72 ± 10.04 | 8.85 ± 2.34 | 11.65 ± 2.63 | 0.223 |
| Acetylcholine | 26.33 ± 12.51 a | 20.06 ± 4.84 a,b | 14.41 ± 0.95 b | 0.008 |
| Succinate | 14.19 ± 9.50 | 13.18 ± 5.86 | 7.93 ± 2.78 | 0.096 |
| Carnitine | 8.96 ± 3.06 | 6.67 ± 1.51 | 8.26 ± 1.23 | 0.060 |
| Unknown 1 | 6.79 ± 2.58 | 7.33 ± 3.44 | 5.75 ± 0.46 | 0.371 |
| Creatine | 394.25 ± 92.32 a | 416.88 ± 81.01 a | 257.69 ± 19.67 b | <0.001 |
| Phosphocreatine | 11.52 ± 2.96 b | 11.48 ± 2.50 b | 23.24 ± 5.54 a | <0.001 |
| Trimethylamine-N-oxide | 30.17 ± 15.01 b | 45.40 ± 22.90 a | 25.20 ± 6.98 b | 0.027 |
| Unknown 2 | 3.79 ± 1.45 a | 2.55 ± 1.07 b | 4.61 ± 1.41 a | 0.006 |
| 3-Hydroxyisobutyrate | 11.75 ± 1.79 | 10.54 ± 2.30 | 8.25 ± 0.83 | 0.117 |
| Glycine | 12.97 ± 5.91 | 12.07 ± 7.16 | 13.64 ± 2.17 | 0.816 |
| β-hydroxypyruvate | 17.84 ± 1.79 a | 17.89 ± 2.30 a | 14.18 ± 0.83 b | <0.001 |
| Unknown 3 | 2.68 ± 0.74 b | 3.51 ± 0.95 a | 2.16 ± 0.74 b | 0.004 |
| Adenosine diphosphate | 4.25 ± 1.95 a | 4.63 ± 1.27 a | 1.31 ± 0.32 b | <0.001 |
| Adenine | 8.31 ± 4.40 b | 8.72 ± 2.92 b | 18.20 ± 1.57 a | <0.001 |
| Unknown 4 | 6.71 ± 0.77 b | 9.33 ± 2.17 a | 8.70 ± 2.07 a | 0.008 |
| Breeds | Metabolites | VIP | p-Value | Coefficient | FDR |
|---|---|---|---|---|---|
| TN vs. BT | Unknown 4 | 2.4946 | 0.002 | −0.7669 | 0.043 |
| TN vs. CB | Valine | 1.5107 | <0.001 | −0.9001 | <0.001 |
| Leucine | 1.4722 | <0.001 | −0.8771 | <0.001 | |
| Phosphocreatine | 1.4166 | <0.001 | −0.8440 | <0.001 | |
| Adenine | 1.4126 | <0.001 | −0.8416 | <0.001 | |
| β-hydroxypyruvate | 1.3993 | <0.001 | 0.8337 | <0.001 | |
| Ethanol | 1.3449 | <0.001 | 0.8013 | <0.001 | |
| Adenosine diphosphate | 1.2807 | <0.001 | 0.7631 | <0.001 | |
| Creatine | 1.2655 | <0.001 | 0.7540 | <0.001 | |
| Acetylcholine | 1.1157 | 0.003 | 0.6647 | <0.001 | |
| Lactate | 1.0973 | 0.008 | 0.6538 | 0.016 | |
| BT vs. CB | Leucine | 1.3805 | <0.001 | 0.9107 | <0.001 |
| Adenine | 1.3545 | <0.001 | 0.8936 | <0.001 | |
| Adenosine diphosphate | 1.3480 | <0.001 | −0.8893 | <0.001 | |
| Valine | 1.3396 | <0.001 | 0.8837 | <0.001 | |
| Ethanol | 1.2745 | <0.001 | −0.8407 | <0.001 | |
| Creatine | 1.2687 | <0.001 | −0.8369 | <0.001 | |
| Phosphocreatine | 1.2681 | <0.001 | 0.8365 | <0.001 | |
| β-hydroxypyruvate | 1.1678 | <0.001 | −0.7704 | <0.001 | |
| Lactate | 1.1131 | <0.001 | −0.7343 | <0.001 | |
| Unknown 2 | 1.0333 | 0.002 | 0.6817 | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phoemchalard, C.; Uriyapongson, S.; Tathong, T.; Pornanek, P. 1H NMR Metabolic Profiling and Meat Quality in Three Beef Cattle Breeds from Northeastern Thailand. Foods 2022, 11, 3821. https://doi.org/10.3390/foods11233821
Phoemchalard C, Uriyapongson S, Tathong T, Pornanek P. 1H NMR Metabolic Profiling and Meat Quality in Three Beef Cattle Breeds from Northeastern Thailand. Foods. 2022; 11(23):3821. https://doi.org/10.3390/foods11233821
Chicago/Turabian StylePhoemchalard, Chirasak, Suthipong Uriyapongson, Tanom Tathong, and Pitukpol Pornanek. 2022. "1H NMR Metabolic Profiling and Meat Quality in Three Beef Cattle Breeds from Northeastern Thailand" Foods 11, no. 23: 3821. https://doi.org/10.3390/foods11233821